Abstract
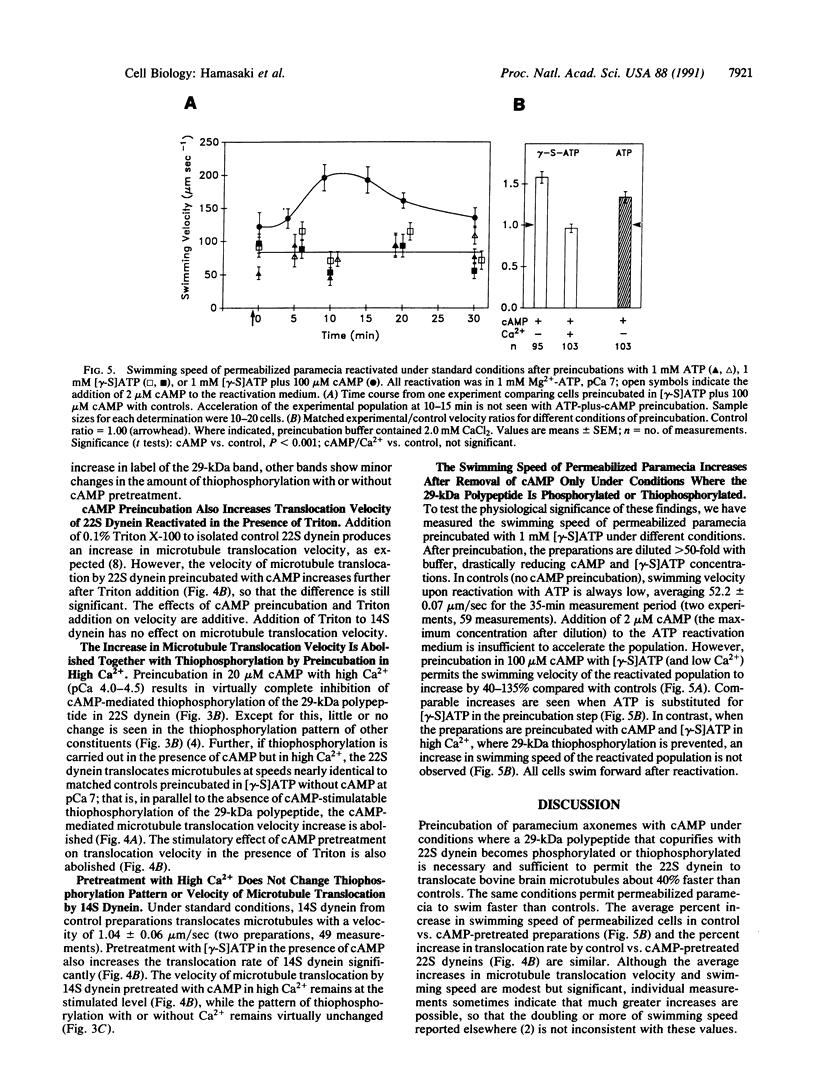
In Paramecium tetraurelia, cyclic nucleotides are important physiological second messengers that could regulate dynein mechanochemistry by phosphorylation. A 29-kDa polypeptide that is phosphorylated in a cAMP- and Ca(2+)-sensitive manner in permeabilized cells and isolated axonemes is the only significant phosphorylated moiety that consistently copurifies with 22S dynein from paramecium cilia. It is not a component of 14S dynein. This polypeptide can be thiophosphorylated in a cAMP-sensitive manner, and this form of 22S dynein is stable when stored at -70 degrees C. cAMP-mediated thiophosphorylation of the 29-kDa polypeptide significantly increases the velocity with which 22S dynein causes microtubules to glide in vitro. The increase is abolished, together with the thiophosphorylation of the 29-kDa polypeptide, by preincubation with high Ca2+. Pretreatment with high Ca2+ does not alter the thiophosphorylation pattern of, or the velocity of microtubule translocation by, 14S dynein. The same preincubation conditions that permit or abolish the increase in velocity of microtubule translocation by 22S dynein permit or fail to permit swimming speed of permeabilized cells to increase on reactivation even after cAMP is removed. The effect of cAMP on swimming speed can therefore be accounted for by changes in the mechanically coupled 22S dynein activity via phosphorylation or thiophosphorylation of the 29-kDa polypeptide, which could act as a regulatory dynein light chain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonini N. M., Evans T. C., Miglietta L. A., Nelson D. L. The regulation of ciliary motility in Paramecium by Ca2+ and cyclic nucleotides. Adv Second Messenger Phosphoprotein Res. 1991;23:227–272. [PubMed] [Google Scholar]
- Bonini N. M., Gustin M. C., Nelson D. L. Regulation of ciliary motility by membrane potential in Paramecium: a role for cyclic AMP. Cell Motil Cytoskeleton. 1986;6(3):256–272. doi: 10.1002/cm.970060303. [DOI] [PubMed] [Google Scholar]
- Bonini N. M., Nelson D. L. Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J Cell Biol. 1988 May;106(5):1615–1623. doi: 10.1083/jcb.106.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Nelson D. L. Phosphoproteins associated with cyclic nucleotide stimulation of ciliary motility in Paramecium. J Cell Sci. 1990 Feb;95(Pt 2):219–230. doi: 10.1242/jcs.95.2.219. [DOI] [PubMed] [Google Scholar]
- Hamasaki T., Murtaugh T. J., Satir B. H., Satir P. In vitro phosphorylation of Paramecium axonemes and permeabilized cells. Cell Motil Cytoskeleton. 1989;12(1):1–11. doi: 10.1002/cm.970120102. [DOI] [PubMed] [Google Scholar]
- Larsen J., Barkalow K., Hamasaki T., Satir P. Structural and functional characterization of paramecium dynein: initial studies. J Protozool. 1991 Jan-Feb;38(1):55–61. doi: 10.1111/j.1550-7408.1991.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Lieberman S. J., Hamasaki T., Satir P. Ultrastructure and motion analysis of permeabilized Paramecium capable of motility and regulation of motility. Cell Motil Cytoskeleton. 1988;9(1):73–84. doi: 10.1002/cm.970090108. [DOI] [PubMed] [Google Scholar]
- Nasr A., Satir P. Alloaffinity filtration: a general approach to the purification of dynein and dynein-like molecules. Anal Biochem. 1985 Nov 15;151(1):97–108. doi: 10.1016/0003-2697(85)90058-2. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Bell C. W., Sale W. S., Gibbons I. R. Structure of the dynein-1 outer arm in sea urchin sperm flagella. I. Analysis by separation of subunits. J Biol Chem. 1982 Jan 10;257(1):508–515. [PubMed] [Google Scholar]
- Travis S. M., Nelson D. L. Purification and properties of dyneins from Paramecium cilia. Biochim Biophys Acta. 1988 Jul 14;966(1):73–83. doi: 10.1016/0304-4165(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Toyoshima Y. Y. Microtubule translocation properties of intact and proteolytically digested dyneins from Tetrahymena cilia. J Cell Biol. 1989 Jun;108(6):2327–2334. doi: 10.1083/jcb.108.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]