Abstract
The diamondback moth (DBM), Plutella xylostella (L.), is one of the most serious cruciferous pests and has developed high resistance to most insecticides, including chlorantraniliprole. Previous studies have reported several protein-coding genes that involved in chlorantraniliprole resistance, but research on resistance mechanisms at the post-transcription level is still limited. In this study, a global screen of microRNAs (miRNAs) associated with chlorantraniliprole resistance in P. xylostella was performed. The small RNA libraries for a susceptible (CHS) and two chlorantraniliprole resistant strains (CHR, ZZ) were constructed and sequenced, and a total of 199 known and 30 novel miRNAs were identified. Among them, 23 miRNAs were differentially expressed between CHR and CHS, and 90 miRNAs were differentially expressed between ZZ and CHS, of which 11 differentially expressed miRNAs were identified in both CHR and ZZ. Using miRanda and RNAhybrid, a total of 1,411 target mRNAs from 102 differentially expressed miRNAs were predicted, including mRNAs in several groups of detoxification enzymes. The expression of several differentially expressed miRNAs and their potential targets was validated by qRT-PCR. The results may provide important clues for further study of the mechanisms of miRNA-mediated chlorantraniliprole resistance in DBM and other target insects.
The diamondback moth (DBM), Plutella xylostella (L.) (Lepidoptera: Plutellidae), is a major pest of cruciferous vegetables and is known to cause serious losses in agricultural production. The global control and damage costs for this insect pest are estimated at 4–5 billion dollars per year1. Due to the long-term use of chemical control coupled with the intensive and irrational use of insecticides, P. xylostella has developed resistance to various types of insecticides and has become one of the most resistant pests in the world2.
Chlorantraniliprole is a type of anthranilic diamide insecticide with a unique mode of action that activates the muscle ryanodine receptor (RyR)3. Because of this novel mode of action, chlorantraniliprole is very effective in controlling several orders of insects, especially lepidopteran pests, and shows no cross-resistance to other commonly used insecticides3. However, this insecticide has been applied worldwide since it came on the market, and in recent years, P. xylostella has developed high levels of resistance to chlorantraniliprole in many countries, including China4,5,6,7.
At present, the research on the mechanisms of chlorantraniliprole resistance in insects is mainly focused on target resistance and detoxification metabolisms. The point mutations in P. xylostella RyR8,9,10 and the increased activity of detoxification enzymes including cytochrome P450 monooxygenase (P450), carboxylesterase (CarE) and glutathione S-transferase (GSTs)11,12 have been demonstrated to be responsible for chlorantraniliprole resistance. However, knowledge of the regulation mechanisms of these genes is relatively limited. Most recently, two miRNAs (miR-7a and miR-8519) were found to be involved in chlorantraniliprole resistance through the up-regulation of RyR expression in P. xylostella13, which was the first report of miRNA-mediated chlorantraniliprole resistance in insects.
MiRNA is a type of endogenous, small non-coding RNA that plays important regulatory roles by targeting mRNAs for cleavage or translational repression. These small RNAs are usually 18–25 nt in length, and their precursors, which usually fold into stem-loop structures, are processed by Dicer endonuclease into two mature miRNAs, one from the plus strand and the other from the minus strand (star miRNA or miRNA*); in most cases, the star miRNA is presumed to be degraded14. The mature miRNA is loaded into an RNA-induced silencing complex (RISC), and it then guides the RISC to its specific mRNA target, where the miRNA “seed sequence” (nucleotides 2–8 at the 5′end) binds to the 3′ untranslated regions (3′UTR) of target mRNA, resulting in the repression of mRNA translation in animals or mRNA degradation in plants15,16. Other research has reported that some miRNAs could also bind to the 5′UTR17,18 or the open reading frame19 to suppress the expression of their target mRNAs.
The first miRNA was discovered in Caenorhabditis elegans over two decades ago20. Since then, a large number of miRNAs have been identified in many types of eukaryotes and viruses using a variety of methods.
The first group of miRNAs in P. xylostella was reported in 2013, when a total of 235 miRNAs were identified from second instar larvae under parasitic stress21. That same year, Liang et al. identified 462 miRNAs in all developmental stages of P. xylostella22. Although a number of miRNAs have been discovered in P. xylostella, a systematical identification of miRNAs associated with insecticide resistance in P. xylostella has not yet been conducted.
A laboratory-susceptible P. xylostella strain and two chlorantraniliprole-resistant strains were selected for this study. The global expression profiles of known and novel miRNAs were compared between the susceptible and two resistant strains, respectively, using high-throughput sequencing, and a batch of miRNAs associated with chlorantraniliprole resistance was obtained. We also predicted the targets of differentially expressed miRNAs by two different algorithms, and the functional annotation of the targets was also performed. These results will be helpful for further study of the role of miRNAs in the regulation of insecticide resistance in P. xylostella.
Results
Chlorantraniliprole resistance levels in CHS, CHR and ZZ
Chlorantraniliprole toxicity to different strains of P. xylostella is summarized in Table 1. The LC50 values of chlorantraniliprole for CHS, CHR and ZZ were 0.112 mg L−1, 5.097 mg L−1 and 4.681 mg L−1, respectively. That is, the resistance ratios for CHR and ZZ were 45.5- and 41.8- fold, respectively (Table 1).
Table 1. Toxicity of chlorantraniliprole to different strains of Plutella xylostella.
| Strain | Numbera | LC50 (mg L−1) (95% CLb) | Slope ± SE | χ2 (df)c | RR at LC50d |
|---|---|---|---|---|---|
| CHS | 351 | 0.112 (0.099–0.125) | 3.172 ± 0.348 | 7.292 (13) | - |
| CHR | 357 | 5.097 (4.586–5.573) | 4.292 ± 0.484 | 7.958 (13) | 45.51 |
| ZZ | 360 | 4.681 (4.238–5.107) | 4.345 ± 0.376 | 8.817 (13) | 41.79 |
aNumber of larvae assayed; bConfidence limits; cChi-square value (χ2) and degrees of freedom (df) as calculated by PoloPlus; dRR: Resistance ratio = LC50 of CHR or ZZ/LC50 of CHS.
Sequencing of miRNAs from P. xylostella
Three small RNA (sRNA) libraries for CHS, CHR and ZZ were constructed, and 45,297,627, 47,566,639 and 40,906,561 raw reads were generated from the sRNA library for CHS, CHR and ZZ, respectively. The low-quality sequences, reads without a 3′ adaptor and reads that were less than 18 nt were eliminated; subsequently, 31,952,995, 33,561,726, and 29,46,332 clean reads were respectively obtained for the three strains and used for further analysis (Table 2). The length distributions are displayed in Fig. 1. The three libraries shared a similar distribution pattern, with 25 nt sRNAs being the most abundant, followed by 24, 26, 23 and 22 nt (Fig. 1).
Table 2. Categorization and abundance of sRNA reads from CHS, CHR and ZZ.
| Data Processing/Strain | Reads all | Reads unique | ||||
|---|---|---|---|---|---|---|
| CHS | CHR | ZZ | CHS | CHR | ZZ | |
| Raw reads | 45297627 | 47566639 | 40906561 | 5066237 | 5421221 | 4643140 |
| Clean reads | 31952995 | 33561726 | 29468332 | 4076241 | 4431220 | 3484682 |
| Mapped to P. xylostella miRNAs reported by Etebari et al. or Liang et al. in 201322 | 1400099 (4.38%) | 1448802 (4.31%) | 621992 (2.20%) | 1595 (0.04%) | 1602 (0.03%) | 1350 (0.04%) |
| Mapped to other RNAs (RFam: rRNA, tRNA, snRNA, snoRNA and others) | 7781970 (24.35%) | 7883299 (23.49%) | 7968865 (27.04%) | 314018 (7.70%) | 313629 (7.08%) | 311243 (8.93%) |
| Mapped to known P. xylostella genes | 1427410 (4.47%) | 1199346 (3.57%) | 552586 (1.88%) | 483528 (11.86%) | 434144 (9.80%) | 222222 (6.38%) |
| Mapped to Repbase | 542288 (1.70%) | 504062 (1.50%) | 501382 (1.70%) | 23230 (0.57%) | 20743 (0.47%) | 20311 (0.58%) |
| Unannotated Sequences | 20801228 (65.10%) | 22526217 (67.12%) | 19823507 (67.27%) | 3253872 (79.83%) | 3661102 (82.62%) | 2929556 (84.07%) |
Figure 1. Size distribution of small RNAs in CHS, CHR and ZZ libraries.
Identification of known miRNAs
Before this research, 235 miRNAs and 462 miRNAs in P. xylostella had already been identified by Etebari et al.21 and Liang et al.22, respectively, in 2013. However, a number of same or similar miRNAs in these two publications were named differently. Therefore, we collated all the miRNAs reported in these two references first, and then all clean sequences generated from this study were mapped against the resulting miRNAs. As a result, 199 known mature miRNA were obtained. Then the pre-miRNA sequences of these mature miRNAs were aligned to those reported by Etebari et al.21 and Liang et al.22 and mapped to the latest version of P. xylostella genome, and 121 confident pre-miRNA sequences were conformed, which produced 172 of 199 identified mature miRNAs (Table S1). However, the pre-miRNA sequences of the rest 27 conserved miRNAs were not detected in the current P. xylostella genome. Considering the incomplete assembly of this DBM genome version, these 27 conserved miRNAs were also used for further analysis (Table S2).
Identification of novel miRNAs
After removal of the identified known miRNA sequences mentioned above, the rest of the clean sequences were processed to remove any non-coding RNAs, protein-coding RNA fragments and repeated sequences. Finally, 20,801,228 (CHS), 22,526, 217 (CHR) and 19,823,507 (ZZ) unannotated clean sequences were obtained and used to predict novel miRNAs (Table 2).
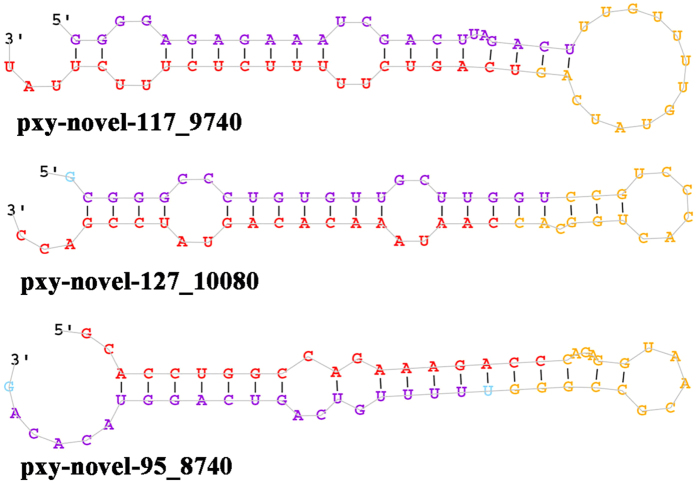
According to the unannotated sequences, miRNA precursor sequences and structures were predicted and identified using miRDeep2 (a probabilistic algorithm based on the miRNA biogenesis model)23, and a total of 51 potential novel miRNAs were initially predicted from the three libraries (Table S3). RNAfold was used to confirm the structures of the predicted miRNAs24. After keeping only novel miRNAs with a rand fold P-value ≤ 0.05 and a miRDeep2 score ≥3, 30 potential novel microRNAs were retained and used for expression analysis, and 24 were determined to have complementary star miRNA sequences (most of the star miRNAs have a low copy number). The length of the novel miRNAs ranged from 18 to 25 nt. Novel miRNAs were named based on their positions in the P. xylostella genome (Table S3). Secondary structures for some potential miRNA precursors with high miRDeep2 scores are shown in Fig. 2.
Figure 2. Predicted secondary structure of three selected novel miRNAs.
The entire sequence represents pre-miRNAs, the red represents mature miRNA, and the purple represents miRNA*.
The most abundant miRNAs in P. xylostella
MiR-Bantam, miR-10 and miR-281 were the three most abundant miRNAs in each of the three libraries. These three miRNAs were also highly expressed in P. xylostella second instar larvae reported by Etebari et al.21. Ten of the most highly expressed miRNAs are listed in Table 3, and a total of 20 miRNAs had high expression with a mean count number >10,000, including 2 novel miRNAs, pxy-novel-117_9740 and pxy-novel-95_8740 (Tables S1, S2, S3).
Table 3. 10 of most highly expressed miRNAs in P. xylostella small RNA libraries.
| MiRNA | Absolute read counts | Mature sequence | ||
|---|---|---|---|---|
| CHS | CHR | ZZ | ||
| pxy-bantam-3p | 268877 | 285557 | 99336 | UGAGAUCAUUGUGAAAGCUGAU |
| pxy-miR-10-5p | 177805 | 204671 | 88001 | UACCCUGUAGAUCCGAAUUUGU |
| pxy-miR-281-5p | 170250 | 160753 | 76068 | AAGAGAGCUAUCCGUCGACAGUA |
| pxy-miR-8-3p | 145792 | 155955 | 52901 | UAAUACUGUCAGGUAAAGAUGUC |
| pxy-miR-31-5p | 78039 | 82910 | 40113 | AGGCAAGAUGUCGGCAUAGCUGA |
| pxy-miR-184-3p | 57775 | 59141 | 34587 | UGGACGGAGAACUGAUAAGGGC |
| pxy-miR-263a-5p | 56688 | 55596 | 31236 | AAUGGCACUGAAAGAAUUCACGGG |
| pxy-miR-6094-3p | 50483 | 46670 | 25105 | UAUUCGAGACCUCUGCUGAUCCU |
| pxy-miR-279b-3p | 32917 | 39015 | 11240 | UGACUAGAUUUUCACUCAUCCUA |
| pxy-miR-9b-5p | 31184 | 29007 | 13145 | UCUUUGGUAUUCUAGCUGUAG |
Analysis of differentially expressed miRNAs
To systematically identify chlorantraniliprole resistance associated miRNAs, a differential expression analysis was performed among the three strains using the sequencing results. In total, 20 known and 3 novel miRNAs were identified as differentially expressed between CHR and CHS; 13 were significantly down-regulated, and 10 were significantly up-regulated (Table S4, Fig. 3A). In addition, 80 known and 10 novel miRNAs were differentially expressed between ZZ and CHS, 89 were significantly down-regulated, and only 1 were up-regulated (Table S5, Fig. 3B). Compared to CHS, 9 known and 2 novel miRNAs were found to be differentially expressed in both CHR and ZZ, 10 were down-regulated in both resistant strains, except pxy-miR-8491-5p, which was up-regulated (Fig. 3C, Table 4). Overall, most of the differentially expressed miRNAs were down-regulated in the resistant strains.
Figure 3. Differentially expressed miRNAs identified among CHS, CHR and ZZ.
(A) Scatter plot of differentially expressed miRNAs between CHS and CHR; (B) Scatter plot of differentially expressed miRNAs between CHS and ZZ. Each point represents a miRNA. Red points indicate a fold change >1. Black points indicate −1< fold change <1. Green points indicate a fold change <−1; (C) Number of differentially expressed miRNAs among CHS, CHR and ZZ.
Table 4. Common differentially expressed miRNAs in CHR and ZZ compared to CHS.
| MiRNA | CHR/CHS Log2 ratio | ZZ/CHS Log2 ratio | UP_ down |
|---|---|---|---|
| pxy-mir-8487-3p | −1.20 | −1.97 | Down |
| pxy-miR-8488-5p | −2.66 | −4.23 | Down |
| pxy-miR-8491-5p | 1.60 | 1.66 | Up |
| pxy-miR-8533-3p | −1.01 | −1.41 | Down |
| pxy-miR-8534-5p | −1.66 | −1.79 | Down |
| pxy-miR-375-5p | −1.95 | −2.34 | Down |
| pxy-miR-4969-5p | −1.57 | −1.76 | Down |
| pxy-miR-625_85053-3p | −1.40 | −1.91 | Down |
| pxy-miR-64_964078-5p | −1.78 | −1.72 | Down |
| pxy-novel-13_1575 | −1.56 | −3.69 | Down |
| pxy-novel-293_16368-5p | −2.39 | −1.79 | Down |
Target prediction and annotation of differentially expressed miRNAs
Usually, miRNA functions by binding to its target mRNAs, therefore annotating the potential targets of differentially expressed miRNAs, and is very important in defining their roles in chlorantraniliprole resistance.
For 23 significantly differentially expressed miRNAs between CHS and CHR, a total of 5,384 targets were predicted using miRanda, and 5,241 targets were identified using RNAHybird (Table S6, Fig. 4A). Similarly, for 90 significantly differentially expressed miRNAs between CHS and ZZ, 23,129 and 20,111 targets were predicted with miRanda and RNAHybird, respectively (Table S7, Fig. 4B).
Figure 4. Identification of potential target genes for differentially expressed miRNAs among CHS, CHR and ZZ using miRanda and RNAhybrid.
(A) Numbers of predicted target genes for the differentially expressed miRNAs between CHS and CHR using miRanda and RNAhybrid; (B) Numbers of predicted target genes for the differentially expressed miRNAs between CHS and ZZ using miRanda and RNAhybrid; (C) Binding site position variation in the two algorithms for the differentially expressed miRNAs between CHS and CHR; (D) Binding site position variation in the two algorithms for the differentially expressed miRNAs between CHS and ZZ.
To make the prediction results more reliable, the miRNA targets predicted by both miRanda and RNAhybrid were considered the final target genes. Finally, 242 miRNA-mRNA pairs between CHS and CHR (Table S8, Fig. 4A), and 1,276 miRNA-mRNA pairs between CHS and ZZ were supported by both algorithms (Table S9, Fig. 4B). Furthermore, all of the binding sites predicted by miRanda and RNAhybrid between miRNAs and their mRNA targets were counted. For different expressed miRNAs and their potential target mRNAs between CHS and CHR, 24% of the binding sites predicted by the two algorithms were almost identical (0 nt difference), and only 7% showed shifts more than 5 nt (Fig. 4C). Similarly, for different expressed miRNAs and their potential target mRNAs between CHS and ZZ, 23% of the binding sites predicted by the two algorithms were almost identical, and 9% showed shifts more than 5 nt (Fig. 4D).
A set of miRNAs were found to target several families of important genes that are often involved in insecticide resistance, such as cytochrome P45025,26,27,28, esterase29, GSTs30, ABC transporter family protein31,32, cuticle protein33, glutamate-gated chloride channel34,35 and superoxide dismutase (SOD)36. The target genes of some selected miRNAs are listed in Table 5. The final predicted targets for the differentially expressed miRNAs were used for qRT-PCR analysis, and their GO annotations and KEGG pathway mapping results are listed in Table S8 and Table S9, respectively.
Table 5. Potential target genes of miRNAs that have already been identified to be involved in insecticide resistance.
| MiRNA | Target id | Annotation | Related insecticide | References |
|---|---|---|---|---|
| pxy-miR-8533-3p | Px003252 | Larval cuticle protein LCP-30 | Thiamethoxam | Pan et al.33 |
| pxy-miR-100-5p | Px013707 | Cytochrome P450 4V2 | Phoxim; Chlorantraniliprole | Gu et al.26; Lin et al.36 |
| Px012816 | Glutamate-gated chloride channel | Fipronil; Avermectins | Ikeda et al.34; Bloomquist et al.35 | |
| pxy-miR-8534-5p | Px005902 | Cytochrome P450 6B6 | Deltamethrin; Chlorantraniliprole | Zhou et al.25; Lin et al.36 |
| pxy-let-7-3p | Px011036 | Glutathione S-transferase T1 | Chlorpyrifos | Qin et al.30 |
| pxy-miR-11-3p | Px017041 | Superoxide dismutase [Cu-Zn] | Chlorantraniliprole; Chlorantraniliprole | Lin et al.36 |
| pxy-miR-2525-3p | Px005902 | Cytochrome P450 6B6 | Deltamethrin | Zhou et al.25 |
| pxy-miR-275-5p | Px014440 | Cytochrome P450 9e2 | Phosphine | Oppert et al.27 |
| Px011552 | Multidrug resistance-associated protein 4 | Pyrethroid; DDT; Lindane; Chlorantraniliprole | Bariami et al.31; Lu et al.32; Lin et al.36 | |
| pxy-miR-1175-5p | Px004046 | Esterase FE4 | Naled | Hsu et al.29 |
| pxy-miR-279c-3p | Px005900 | Cytochrome P450 6B2 | Bacillus thuringiensis BT | Muñoz et al.28 |
Quantitative RT-PCR validation of differentially expressed miRNAs and their potential targets
To validate the expression profiles of the differentially expressed miRNAs identified from the small RNA sequencing, a number of miRNAs were randomly selected for quantitative RT-PCR (qRT-PCR) assays.
Initially, 4 miRNAs (pxy-miR-276-5p, pxy-miR-6498-5p, pxy-miR-8530-5p and pxy-novel-77_7193) differentially expressed only between CHR and CHS (Fig. 5), 4 miRNAs (pxy-miR-750-5p, pxy-miR-210-3p, pxy-miR-306-5p and pxy-miR-965-3p) differentially expressed only between ZZ and CHS (Fig. 6), and 4 common differentially expressed miRNAs (pxy-miR-8491-5p, pxy-miR-4969-5p, pxy-mir-8488-5p and pxy-novel-13_1575) in both CHR and ZZ compared to CHS (Fig. 7) were used for qRT-PCR validation. The expression patterns of all selected miRNAs showed a similar trend between the results of sequencing and qRT-PCR.
Figure 5. qRT-PCR validation of significantly differentially expressed miRNAs between CHS and CHR.
Different lowercase letters (a and b) represent significant differences by t-test (P < 0.05). The same applies below.
Figure 6. qRT-PCR validation of significantly differentially expressed miRNAs between CHS and ZZ.
Figure 7. qRT-PCR validation of common differentially expressed miRNAs in CHR and ZZ compared to CHS.
To analyze the correlation between the expression levels of miRNAs and their potential targets, the relative expression of three miRNAs (pxy-miR-8533-3p, pxy-miR-8534-5p and pxy-miR-375-5p) down-regulated in both CHR and ZZ and their corresponding targets, including larval cuticle protein LCP-30, cytochrome P450 6B6 and cytochrome P450 4G15, were verified through qRT-PCR. The expression of the 3 selected miRNAs were all down-regulated, which shared a similar trend with the sequencing results, while the expression of their corresponding targets were all up-regulated (Fig. 8). All 3 selected miRNAs showed a significant negative correlation with their targets.
Figure 8. qRT-PCR analysis of significantly differentially expressed miRNAs and their potential targets.
Discussion
In recent years, an increasing number of papers on insect miRNA have been published37. A total of 3,824 mature miRNAs belonging to 26 species of insects have already been deposited in miRBase. MiRNA plays very important roles in the growth and development of insects, such as the reproductive process38 germ cell development39 neurogenesis40,41, wing development42,43,44, phenotypic plasticity45 and muscle growth46,47,48. More recently, miRNAs were also found to be involved in insecticide resistance49,50,51.
To systematically identify miRNAs associated with chlorantraniliprole resistance in P. xylostella, small RNAs from a susceptible strain (CHS) and two resistant strains (CHR, ZZ) were sequenced using Illumina sequencing technology in this study. The differentially expressed miRNAs among CHS, CHR and ZZ were analyzed, and their target genes were also predicted. A total of 229 miRNAs were identified in the three libraries, of which 199 miRNAs had already been reported in P. xylostella before, and 30 novel miRNAs were predicted using miRdeep2 for the first time.
Some highly conserved miRNAs, such as miR-let-7, miR-8, miR-9, miR-184, miR-278 and miR-bantam, which play essential roles in many types of insects37, were also discovered with high expression in the three P. xylostella strains, implying important regulatory roles in P. xylostella.
Twenty-three miRNAs were identified to be differentially expressed between CHR and CHS. Because CHR was established from CHS by successive selection with chlorantraniliprole (i.e., they have same genetic background) and have been reared under same laboratory conditions with CHS, the 23 differentially expressed miRNAs are likely to be associated with chlorantraniliprole resistance. Between ZZ and CHS, 90 differentially expressed miRNAs were identified. The ZZ strain was a field strain, and it had developed high levels of resistance to several other commonly used insecticides, such as beta-cypermethrin, abamectin, spinosad and indoxacarb (unpublished data from a local plant protection station), in addition to chlorantraniliprole. Each of these insecticides kills P. xylostella with distinctive modes of action. Therefore, the 90 differentially expressed miRNAs likely result from of the comprehensive effects of these different insecticides as well as other environmental factors.
When the differentially expressed 23 and 90 miRNAs were put together, we found that 11 of them overlapped. The overlapped miRNAs are likely to be involved in chlorantraniliprole resistance because they were differentially expressed in both laboratory-selected and field-collected resistant strains. However, due to the complex insecticide resistance mechanisms in the ZZ strain, the 11 miRNAs may also reveal common resistant mechanisms to other insecticides. In fact, some of them have been reported to be associated with several insecticides in different insects. For example, Hong et al. identified miR-375 and 27 other differentially expressed miRNAs between deltamethrin-susceptible and resistant Culex pipiens strains49. In this study, we found that an analog of miR-375 was differentially expressed between chlorantraniliprole susceptible and the two resistant P. xylostella strains, though with low abundance. These results, together with one P450 mRNA, imply that miR-375 has a high possibility of involvement in the regulation of insecticide resistance. The unique differentially expressed miRNAs in the CHR strain may reveal a unique mechanism of resistance to chlorantraniliprole; therefore, we should pay more attention to these 10 miRNAs in our follow-up work. The unique differentially expressed miRNAs in the ZZ strain, especially those that have different expression profiles compared with CHR, are more likely to be involved in other insecticide resistance and not chlorantraniliprole resistance. Some of them have been reported to be associated with deltamethrin, Cry1Ab or fenpropathrin resistance, such as miR-210, miR-965, miR-981, miR-1, miR-306 and miR-28149,50,51.
Because experimental validation of miRNA targets is still a major challenge, in silico prediction is widely used for the identification of potential miRNA targets52. In the current study, the target genes of the differentially expressed miRNAs among CHS, CHR and ZZ were predicted using two different algorithms, miRanda and RNAhybrid. Only the target genes supported by both algorithms were retained, and thus, the predicted results were relatively more credible. Although we did not find a ryanodine receptor in the predicted results, many other insecticide resistance associated genes were discovered, such as P450, esterase, GSTs, ABC transporter family protein, cuticle protein and SOD genes, of which several cytochrome P450, multidrug resistance-associated protein 4 (ABCC4) and SOD genes have already been identified to be differentially expressed among the susceptible strain and 3 different chlorantraniliprole-resistant P. xylostella strains36. Esterase FE429, GSTT130 and larval cuticle protein LCP-30 genes33 were also confirmed to be involved in resistance to other insecticides. In addition, some other metabolic enzymes were predicted to be associated with the differently expressed miRNAs, but there was no evidence for their involvement in insecticide resistance yet. Interestingly, several of them were discovered to be involved in detoxification process in mammal, such as UDP-glucuronosyltransferase 2A153.
In this study, a number of miRNAs were speculated to be involved in chlorantraniliprole resistance in P. xylostella based only on their differential expression profiles and their predicted target genes. To further reveal the roles of these miRNAs in chlorantraniliprole resistance, gain- and loss-of-function experiments should be carried out in vivo and could include, for example, the up- or down-regulation of the expression of a miRNA by injecting its synthetic mimics or inhibitors and suppressing the expression of its target mRNAs by RNA interference (RNAi). This should be followed by the evaluation of resistance levels in treated larvae using bioassays.
Conclusion
This paper presents the first study to systematically screen miRNAs associated with chlorantraniliprole resistance in P. xylostella. In this study, we identified 199 known miRNAs and 30 novel miRNAs in three DBM strains. A set of differentially expressed miRNAs among CHS, CHR and ZZ were obtained and considered highly likely to be associated with chlorantraniliprole resistance. The potential targets of the differentially expressed miRNAs were also predicted, and many of the target genes were related to detoxification processes. These results may guide us in further investigating the mechanisms of miRNA-regulated chlorantraniliprole resistance and may provide novel insights into resistance management in P. xylostella.
Materials and Methods
Insects
The laboratory susceptible DBM strain (CHS) was collected in the vegetable fields of Beijing and reared in our laboratory without exposure to any insecticide for more than 10 years. The chlorantraniliprole-resistant strain (CHR) was derived from the CHS strain by successive selection with chlorantraniliprole for more than 60 generations, and the field-resistant strain (ZZ) was collected in the vegetable fields of Zhangzhou, Fujian province, in southeastern China in 2015. All stages of P. xylostella were maintained at 27 ± 1 °C, an RH of 50–60% and a photoperiod of 16 h light/8 h dark on radish seedlings (Raphanus sativus L.). P. xylostella adults were provided with 10% (W/V) honey solution and allowed to mate and oviposit on the radish seedlings.
Bioassay
The Leaf-dip method54 was used in this study. Cabbage leaves were dipped in the required chlorantraniliprole concentrations for 10–15 s and then allowed to air dry in the shade. A 0.1% (v/v) Triton X-100 water solution was used as a control. Approximately 20–25 third instar larvae were transferred onto each leaf, and three replications were used for each concentration. The mortality was assessed after four days of treatment. LC50 values were calculated using POLO-Plus 2.0 software (LeOra Software Inc., Berkeley, CA).
Small RNA library construction and sequencing
RNA samples were prepared from the three DBM strains (each sample containing 30–50 third-instar P. xylostella larvae). Trizol Reagent was used to isolate the total RNA from each sample according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). RNA degradation and contamination were assessed on 1% agarose gels, and RNA concentration was measured using the Nano Drop 2000 (Thermo, Wilmington, USA).
Next, 1 μg of total RNA were ligated sequentially with 3′and 5′ adaptors, and RT-PCR was performed using the TruSeq™ SmallRNA Sample Prep Kits (Illumina) for 15 cycles. The resulting ligation PCR products were isolated from a 6% TBE PAGE gel and sequenced using a HiseqXTEN sequencer (Illumina). Small RNA sequencing and bioinformatics analyses were conducted at the OE Biotechnology Company (Shanghai, China).
Analysis of sequencing data
Data files from each of the three libraries were used for analysis. The raw data of this study were deposited in the NCBI Short Read Archive (SRX1968414, SRX1968415 and SRX1952874). Clean reads were screened from the raw data after processing out low-quality reads, adapters, and sequences of fewer than 18 nucleotides. Clean reads were mapped to the P. xylostella genome55 (version 2, http://iae.fafu.edu.cn/DBM/index.php) to analyze the distribution using bowtie software56.
All P. xylostella miRNAs reported by Etebari et al.21 and Liang et al.22 were collated and re-annotated first, then clean sequences generated in this research were used to search against the resulting miRNAs. All miRNAs identified in this step were considered known miRNAs.
All remaining clean sequences were subjected to the Rfam database to remove known noncoding RNA families, including rRNA, scRNA, snoRNA, snRNA and tRNA (http://www.sanger.ac.uk/Software/Rfam/ftp.shtml), and were then searched against known genes of P. xylostella to discard degraded fragments; the search was concluded in RepBase to remove repeated sequences (http://www.girinst.org). Unannotated clean sequences that did not match any of the above databases were further used to analyze and predict novel miRNAs using miRDeep2 software. Both a false positive rate (FPR) and true positive rate (TPR) were used to assess the predicted results, and RNAfold was used to confirm the structures of the predicted miRNAs.
Differential Expression Analysis of miRNAs
The abundance of miRNAs identified in the three libraries was first normalized using the tags per million reads (TPM) method: TPM = (number of mapped reads for each miRNA/total number of mapped reads) ×106. The log2 (TPM ratios) among the three libraries was calculated, and the P-value was calculated using the Audic Claverie statistic. The miRNAs with |log2 (TPM ratios)| ≥ 1 and P-value < 0.05 were regarded as differentially expressed among the three P. xylostella strains.
Target prediction and annotation of differentially expressed miRNAs
MiRNA usually regulates gene expression through binding to the 3′ untranslated region (3′ UTR) of target mRNAs, so 3′ UTR annotation information was first extracted from the genome database of P. xylostella for target prediction. The potential target genes of differentially expressed miRNAs were predicted and analyzed using two different types of software, miRanda57 and RNAhybrid58,59. For each prediction method, high efficacy targets were selected by the following criteria: (1) miRanda: total score ≥ 140, total energy ≤ −25 kcal/mol; (2) RNAhybrid: P-value < 0.05, mfe ≤ −25 kcal/mol.
Verification of differentially expressed miRNAs and their potential targets by quantitative real-time PCR
Quantitative real-time PCR was performed to experimentally validate the relative expression levels of the identified miRNAs and their potential targets. Total RNA was extracted from the same samples used for deep sequencing. The first-strand cDNA of mature miRNA and mRNA were synthesized using a miScript II RT kit (Qiagen, Germany) and a PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara Biotechnology, Dalian, China), respectively, following the manufacturer’s instructions. qRT-PCR analysis was carried out using SYBR Premix Ex Taq (Takara Biotechnology, Dalian, China). Each reaction was performed in an ABI 7500 Real Time PCR system (Applied Biosystems) with three biological replicates. The expression levels for miRNA and mRNA were normalized to U6 snRNA and ribosomal protein L32 mRNA, respectively. The relative expression levels of the miRNAs and targets were calculated using the 2–ΔΔCt method60. All primers used in this study are listed in Table S10.
Additional Information
How to cite this article: Zhu, B. et al. Global identification of microRNAs associated with chlorantraniliprole resistance in diamondback moth Plutella xylostella (L.). Sci. Rep. 7, 40713; doi: 10.1038/srep40713 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB114103) and the National Natural Science Foundation of China (31171873, 31371956 and 31572023).
Footnotes
Author Contributions Conceived and designed the experiments: P.L. and B.Z. Performed the experiments: B.Z. and X.X.L. Analyzed the data: B.Z., Y.L. and X.X.L. Contributed reagents/materials: P.L. and X.G. Wrote the paper: B.Z., P.L. and X.X.L.
References
- Zalucki M. P. et al. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J Econ Entomol 105, 1115–1129 (2012). [DOI] [PubMed] [Google Scholar]
- Furlong M. J., Wright D. J. & Dosdall L. M. Diamondback moth ecology andmanagement: problems, progress, and prospects. Annu Rev Entomol 58, 517–541 (2013). [DOI] [PubMed] [Google Scholar]
- Lahm G. P., Cordova D. & Barry J. D. New and selective ryanodine receptor activators for insect control. Bioorg Med Chem 17, 4127–4133 (2009). [DOI] [PubMed] [Google Scholar]
- Troczka B. et al. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem Molec 42, 873–880 (2012). [DOI] [PubMed] [Google Scholar]
- Wang X. L. & Wu Y. D. High levels of resistance to chlorantraniliprole evolved infield populations of Plutella xylostella. J Econ Entomol 105, 1019–1023 (2012). [DOI] [PubMed] [Google Scholar]
- Wang X. L., Khakame S. K., Ye C., Yang Y. H. &Wu Y. D. Characterisation of field-evolved resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella, from China. Pest Manag Sci 69, 661–665 (2013). [DOI] [PubMed] [Google Scholar]
- Ribeiro L. M.,Wanderley-Teixeira V., Ferreira H. N., Teixeira A. A. & Siqueira H. A. Fitness costs associated with field-evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). B Entomol Res 104, 88–96 (2014). [DOI] [PubMed] [Google Scholar]
- Troczka B. et al. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem Molec 42, 873–880 (2012). [DOI] [PubMed] [Google Scholar]
- Guo L. et al. Functional analysis of a point mutation in the ryanodine receptor of Plutella xylostella (L.) associated with resistance to chlorantraniliprole. Pest Manag Sci 70, 1083–1089 (2014). [DOI] [PubMed] [Google Scholar]
- Guo L. et al. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep 4, 6924 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang H. Y., Ning Y. B., Qiao K. & Wang K. Y. Resistance selection and characterization of chlorantraniliprole resistance in Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 108, 1978–1985 (2015). [DOI] [PubMed] [Google Scholar]
- Jiang W. H. et al. Chlorantraniliprole susceptibility in Leptinotarsa decemlineata in the north Xinjiang Uygur Autonomous Region in China. J Econ Entomol 105, 549–554 (2012). [DOI] [PubMed] [Google Scholar]
- Li X. X. et al. MiRNAs regulated overexpression of ryanodine receptor is involved in chlorantraniliprole resistance in Plutella xylostella (L.) Sci. Rep 5, 14095 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- He L. & Hannon G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5, 522–531 (2004). [DOI] [PubMed] [Google Scholar]
- Wightman B., Ha I. & Ruvkun G. Post transcriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993). [DOI] [PubMed] [Google Scholar]
- Lytle J. R., Yario T. A. & Steitz J. A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA 104, 9667–9672 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orom U. A., Nielsen F. C. & Lund A. H. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 30, 460–471 (2008). [DOI] [PubMed] [Google Scholar]
- Tay Y., Zhang J., Thomson A. M., Lim B. & Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 455, 1124–1128 (2008). [DOI] [PubMed] [Google Scholar]
- Lee R. C., Feinbaum R. L. & Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993). [DOI] [PubMed] [Google Scholar]
- Etebari K., Hussain M. & Asgari S. Identification of microRNAs from Plutella xylostella larvae associated with parasitization by Diadegma semiclausum. Insect Biochem Mol Biol 43(4), 309–318 (2013). [DOI] [PubMed] [Google Scholar]
- Liang P., Feng B., Zhou X. G. & Gao X. W. Identification and developmental profiling of microRNAs in diamondback moth, Plutella xylostella (L.) PLoS ONE 13, 8(11), e78787 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedländer M. R., Mackowiak S. D., Li N., Chen W. & Rajewsky N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 40(1), 37–52 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. et al. ViennaRNA Package 2.0. Algorithms. Mol Biol 6, 26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. J. et al. Expression responses of nine cytochrome P450 genes to xenobiotics in the cotton bollworm Helicoverpa armigera. Pesticide Biochemistry and Physiology 97(3), 209–2139 (2010). [Google Scholar]
- Gu Z. Y. et al. Differentially expressed genes in the fat body of Bombyx mori in response to phoxim insecticide. Pesticide Biochemistry and Physiology 117, 47–53 (2015). [DOI] [PubMed] [Google Scholar]
- Oppert B. et al. Genes related to mitochondrial functions are differentially expressed in phosphine-resistant and -susceptible Tribolium castaneum. BMC Genomics 16, 968 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz P., López C., Moralejo M., Pérez-Hedo M. & Eizaguirre M. Response of last Instar Helicoverpa armígera larvae to Bt toxin ingestion: changes in the development and in the CYP6AE14, CYP6B2 and CYP9A12 gene expression. PLoS ONE. 9(6), e99229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. K., Huang L. H., Geib S. M. & Hsu J. C. Identification of a carboxylesterase associated with resistance to naled in Bactrocera dorsalis (Hendel). Pestic Biochem Physiol. 131, 24–31 (2016). [DOI] [PubMed] [Google Scholar]
- Qin G. H. et al. Effects of chlorpyrifos on glutathione S-transferase in migratory locust, Locusta migratoria. Pesticide Biochemistry and Physiology 109, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- Bariami V., Jones C. M., Poupardin R., Vontas J. & Ranson H. Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl. Trop. Dis. 6, e1692 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Long Y., Lin L., Sun R. Z., Zhong S. & Cui Z. B. Characterization of zebrafish abcc4 as an efflux transporter of organochlorine pesticides. PLoS ONE. 9(12), e111664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. et al. Transcriptomic comparison of thiamethoxam-resistance adaptation in resistant and susceptible strains of Aphis gossypii Glover. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 13, 10–15 (2015). [DOI] [PubMed] [Google Scholar]
- Ikeda T., Zhao X., Kono Y., Yeh J. Z. & Narahashi T. Fipronil modulation of glutamate-induced chloride currents in cockroach thoracic ganglion neurons. Neuro Toxicology. 24, 807–815 (2003). [DOI] [PubMed] [Google Scholar]
- Bloomquist J. R. Chloride channels as tools for developing selective insecticides. Arch Insect Biochem Physiol. 54, 145–156 (2003). [DOI] [PubMed] [Google Scholar]
- Lin Q. S. et al. Transcriptome analysis of chlorantraniliprole resistance development in the diamondback moth Plutella xylostella. PLoS ONE 8, e72314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S. MicroRNA functions in insects. Insect Biochem Mol Biol 43, 388–397 (2013). [DOI] [PubMed] [Google Scholar]
- Lucasa K. J., Roy S., Haa J., Gervaisea A. L., Kokozaa V. A. & Raikhela A. S. MicroRNA-8 targets the wingless signaling pathway in the female mosquito fat body to regulate reproductive processes. Proc Natl Acad Sci USA 112(5), 1440–1445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovino N., Pane A. & Gaul U. MiR-184 has multiple roles in Drosophila female germline development. Dev. Cell 17, 123–133 (2009). [DOI] [PubMed] [Google Scholar]
- Karres J. S., Hilgers V., Carrera I., Treisman J. & Cohen S. M. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell 131, 136–145 (2007). [DOI] [PubMed] [Google Scholar]
- Loya C. M., Lu C. S., Van Vactor D. & Fulga T. A. Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms. Nat Methods 6, 897–903 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caygill E. E. & Johnston L. A. Temporal regulation of metamorphic processes in Drosophila by the let-7 and miR-125 heterochronic microRNAs. Curr Biol 18, 943–950 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano F., Smibert P. & Lai E. C. MiR-9a prevents apoptosis during wing development by repressing Drosophila LIM-only. Dev Biol 338, 63–73 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukova I., Asmar J., Abdesselem H. & Heitzler P. Drosophila mir-9a regulates wing development via fine-tuning expression of the LIM only factor, dLMO. Dev Biol 327, 486–496 (2009). [DOI] [PubMed] [Google Scholar]
- Wei Y., Chen S., Yang P., Ma Z. & Kang L. Characterization and comparative profiling of the small RNA transcriptomes in two phases of locust. Genome Biol 10, R6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla G. & Sokol N. S. MicroRNAs in Drosophila development. Int Rev Cell Mol Biol 286, 1–65 (2011). [DOI] [PubMed] [Google Scholar]
- Nguyen H. T. & Frasch M. MicroRNAs in muscle differentiation: lessons from Drosophila and beyond. Curr Opin Genet Dev 16, 533–539 (2006). [DOI] [PubMed] [Google Scholar]
- Sokol N. S. & Ambros V. Mesodermally expressed Drosophila microRNA-1 is regulated by Twist and is required in muscles during larval growth. Genes Dev 19, 2343–2354 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. C. et al. Identification of differentially expressed microRNAs in Culex pipiens and their potential roles in pyrethroid resistance. Insect Biochem Mol Biol 55, 39–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. N. et al. Identification of differentially expressed microRNAs between Bacillus thuringiensis Cry1Ab resistant and -susceptible strains of Ostrinia furnacalis. Sci. Rep 5, 15461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. C. et al. Identification of differentially expressed microRNAs between the fenpropathrin resistant and susceptible strains in Tetranychus cinnabarinus. PLoS ONE 10, 1371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W. & Wang X. H. Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Res 34(5), 1646–1652 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M. The Human UDP-Glucuronosyltransferase UGT2A1 and UGT2A2 Enzymes Are Highly Active in Bile Acid Glucuronidation. Drug Metabolism and Disposition 41(9), 1616–1620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Gao X. W. & Zheng B. Z. Genetic basis of resistance and studies on cross-resistance in a population of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag Sci 59, 1232–1236 (2003). [DOI] [PubMed] [Google Scholar]
- You M. S. et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat Genet 45, 220–225 (2013). [DOI] [PubMed] [Google Scholar]
- Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nature methods 9(4), 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright A. J. et al. MicroRNA targets in Drosophila. Genome Biol 5(1), R1–10 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J. & Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res 34, W451–W454 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehmsmeier M., Steffen P., Hochsmann M. & Giegerich R. Fast and effective prediction of microRNA/target duplexes. RNA 10(10), 1507–1517 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.