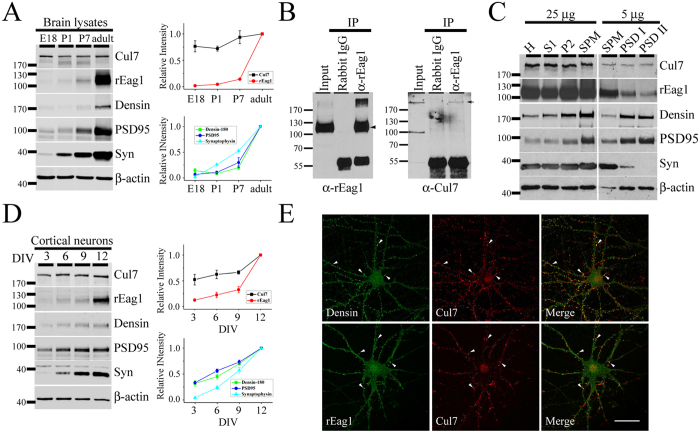
Figure 2. Association of rEag1 with Cul7 in neurons.
(A) (Left) Developmental protein expression patterns of Cul7, rEag1, densin-180, PSD95, synaptophysin (Syn), and β-actin in brain lysates prepared from embryonic day 18 (E18), postnatal days 1 and 7 (P1, P7), and adult rats. (Right) Densitometric quantification of protein signals was standardized as the ratio to the cognate β-actin signals, followed by normalization to the adult group. Data were compiled from 3–5 independent experiments. (B) Co-immunoprecipitation of endogenous rEag1 and Cul7 in the brain. Rat forebrain lysates were immunoprecipitated with the anti-rEag1 antibody or the rabbit IgG, followed by immunoblotting with the indicated antibodies. The protein bands corresponding to rEag1 and Cul7 are highlighted with arrowhead and arrow, respectively. (C) Co-localization of rEag1 and Cul7 at pre- and post-synaptic compartments. Subcellular fractionation separated rat brains into homogenate (H), soluble (S1), crude membrane (P2), synaptosomal (SPM), and two postsynaptic density (PSD I: one Triton X-100 wash; PSD II: two Triton X-100 washes) fractions, all of which were subject to immunoblotting analyses with the indicated antibodies. 25 μg and 5 μg refer to the amount of total protein loaded in each lane. (D) (Left) Developmental protein expression patterns of Cul7, rEag1, densin-180, PSD95, synaptophysin, and β-actin in cultured hippocampal neurons with different days in vitro (DIV). (Right) Densitometric quantification of protein signals was standardized as the ratio to the cognate β-actin signals, followed by normalization to the DIV12 group. Data were compiled from 3 independent experiments. (E) Representative immunofluorescence images of rEag1, Cul7, and densin-180 in DIV12 hippocampal neurons. (Upper panels) Confocal micrographs showing the distribution of densin-180 (green) and Cul7 (red) puncta (arrowheads) along neurites and somas. (Lower panels) Confocal micrographs showing the co-localization of rEag1 (green) and Cul7 (red) puncta (arrowheads) along neurites. Co-localization of red and green puncta (yellow) is indicated in the merge images (see Suppl. Methods and Suppl. Fig. S2A for further quantitative analyses). These studies were reproduced in 3 independent experiments. Scale bar: 25 μm. The gels were run under the same experimental conditions. Uncropped images of immunoblots are shown in Supplementary Figure S8.