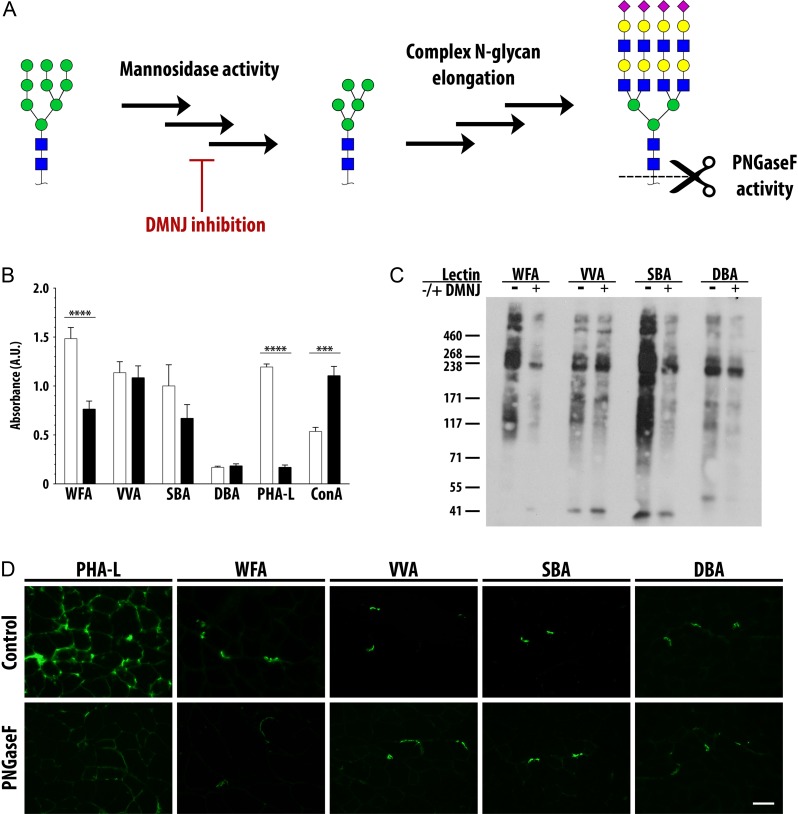
Fig. 5.
Complex N-glycans are required for binding of WFA. (A) High mannose N-glycans present on glycoproteins are trimmed to truncated mannose structures by mannosidase enzyme activity, a process inhibited by the small molecule DMNJ. Glycosyltransferase activity elongates truncated mannose structures into complex N-glycans. The bacterial enzyme PNGaseF cleaves N-glycans between the asparagine and GlcNAc residues. (B) C2C12 myotubes were treated with DMNJ or vehicle control for 72 h. DMNJ treatment abrogated WFA binding and modestly reduced binding of SBA. (C) Equal amounts of lysates from murine C2C12 myotubes treated with DMNJ or vehicle control for 72 h were precipitated by WFA, VVA, SBA and DBA following surface biotinylation and cell lysis. Precipitated proteins were separated by SDS-PAGE, and blots probed with SA-HRP. (D) Eight-micrometer serial quadriceps cryosections from wild-type mice were incubated with PNGaseF or vehicle control for 4 h to enzymatically remove N-glycans. PNGaseF treatment abrogated binding of PHA-L and reduced binding of WFA. NMJs were co-stained with Texas Red conjugated alpha-bungarotoxin. Scale bar is 50 µm. Data are mean ± SEM (error bars) of triplicates from three pooled independent experiments. Monosaccharide symbols follow the SNFG (Symbol Nomenclature for Glycans) system (PMID 26543186, Glycobiology 25: 1323–1324, 2015) details at NCBI. *** P < 0.001; **** P < 0.0001. This figure is available in black and white in print and in color at Glycobiology online.