FIGURE 1.
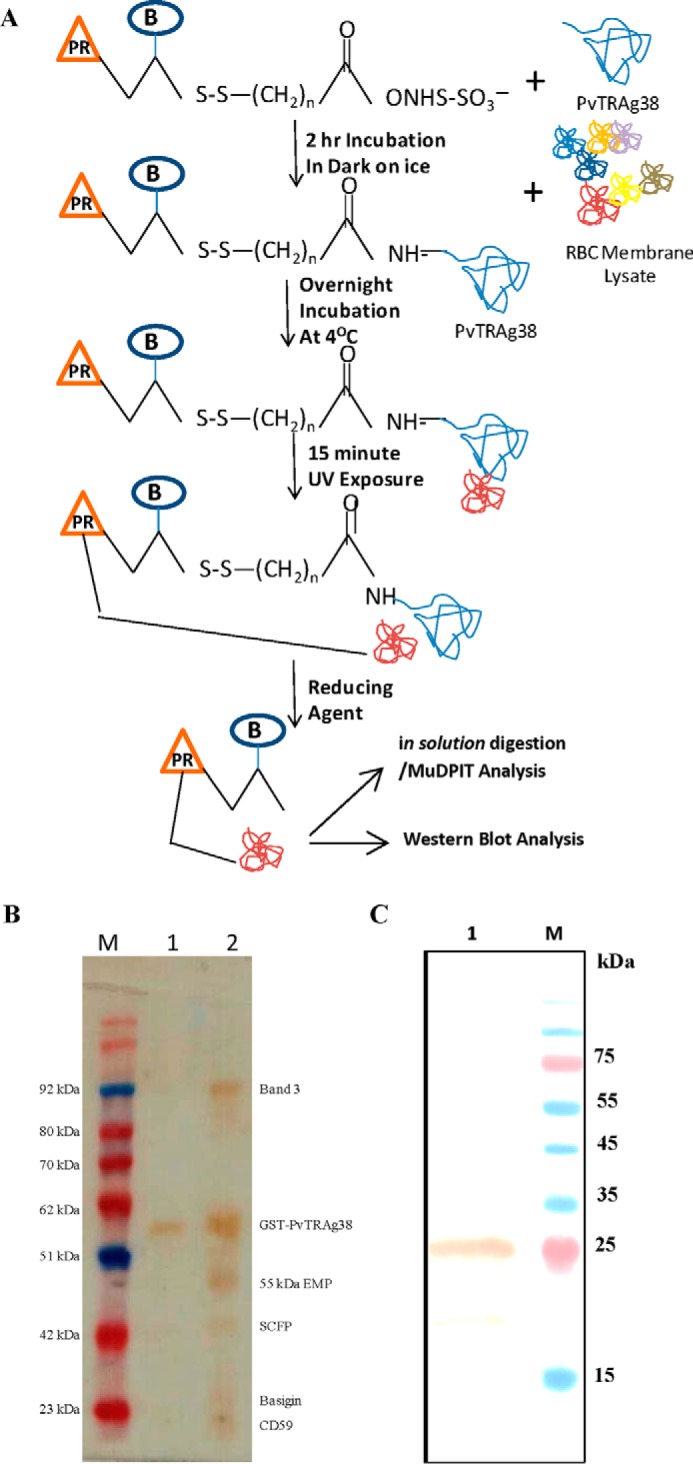
A, schematic diagram depicting the Label transfer method. The GST-tagged PvTRAg38 was incubated with biotin-labeled sulfo-SBED cross-linker. The labeled protein was incubated with erythrocyte membrane proteins, followed by exposure to UV light that photoactivates a photoreactive moiety (represented by orange triangle PR) of trifunctional cross-linker, which will bind to the interacting protein. The Label transfer (represented by blue circle B) was completed by cleaving the spacer arm to release the bait protein by reducing it with DTT. The protein-protein interaction between PvTRAg38 and erythrocyte membrane proteins was detected either by MuDPIT analysis from eluates or by probing eluates with streptavidin-HRP-based Western blot. B, identification of erythrocyte membrane proteins interacting with recombinant PvTRAg38 during Label transfer assay. Erythrocyte membrane proteins extracted with 1% C12E8 were incubated with recombinant GST-tagged PvTRAg38 (labeled with trifunctional Sulfo-SBED cross-linker), washed and resolved on 15% SDS-PAGE, and transferred to nitrocellulose membrane. The blot was probed with streptavidin-HRP. Lane M, molecular weight marker. Lane 1, recombinant GST-tagged PvTRAg38 (labeled with biotin-labeled cross-linker). Lane 2, erythrocyte membrane extract incubated with labeled GST-tagged PvTRAg38. Label transferred to erythrocyte proteins in lane 2 are named on right-hand side. SCFP1, solute-carrier family protein 1. C, detection of basigin in eluates by Western blotting analysis. Eluate was from the Label transfer assay where labeled GST-tagged PvTRAg38 was incubated with reticulocyte-enriched RBCs; membrane lysate was resolved on 15% SDS-PAGE, and protein bands were transferred to nitrocellulose membrane. The blot was probed with anti-basigin antibody (lane 1). Lane M, molecular weight marker lane. Sizes of marker proteins are shown on right-hand side.
