Abstract
Intestinal ischemia/reperfusion (I/R) injury is a relatively common pathological condition that can lead to multi-organ failure and mortality. Regulatory mechanism for this disease is poorly understood, although it is established that circulating pathogenic natural IgM, which is primarily produced by B1a cells outside of the peritoneal cavity, are integrally involved. CD6 was originally identified as a marker for T cells and was later found to be present on some subsets of B cells in humans; however, whether CD6 plays any role in intestinal I/R-induced injury and, if so, the underlying mechanisms, remain unknown. Here we report that CD6−/− mice were significantly protected from intestinal inflammation and mucosal damage compared with WT mice in a model of intestinal I/R-induced injury. Mechanistically, we found that CD6 was selectively expressed on B1 cells outside of the bone marrow and peritoneal cavity and that pathogenic natural IgM titers were reduced in the CD6−/− mice in association with significantly decreased B1a cell population. Our results reveal an unexpected role of CD6 in the pathogenesis of intestinal IR-induced injury by regulating the self-renewal of B1a cells.
Keywords: animal model, immunology, inflammation, innate immunity, intestine
Introduction
The intestine is probably the most sensitive internal organs for ischemia/reperfusion (I/R)2-induced injury (1). Intestinal I/R injury often occurs during abdominal and thoracic vascular surgery, intestinal transplantation, hemorrhagic shock, and surgery using cardiopulmonary bypass. Without intensive management, it leads to intestinal damage, sepsis, systemic inflammation, multiple-organ failure, and eventually death (2). Currently available options for preventing or treating intestinal I/R induced injury are limited; therefore, understanding the regulatory mechanisms would help to develop novel therapies for this life-threatening condition (1–3).
Many inflammatory cytokines contribute to the breach of the gut epithelial barrier during intestinal I/R-induced injury. Among them, IL-6 locally produced in the intestine has been identified as a key inflammatory cytokine that plays an essential role in damaging the tissue during intestinal I/R. In support of this, it has been demonstrated that 1) both in humans (4) and in mice (5, 6), local levels of IL-6 in the intestine are markedly elevated in association with severe tissue damage after intestinal I/R; 2) IL-6−/− mice developed significantly attenuated intestinal damage after I/R; and 3) blocking IL-6 activity in WT mice using anti-IL-6 mAbs greatly retained intestinal tract integrity after I/R (7).
Natural IgM has been found to be critical in the pathogenesis of intestinal I/R. These natural antibodies recognize antigens exposed on hypoxic cells after intestinal ischemia and activate complement through the lectin pathway to produce C5a, which can facilitate neutrophil and monocyte infiltration and inflammatory cytokine (e.g. IL-6) production (8, 9). The first evidence indicating that natural IgM initiates the inflammatory processes to induce intestinal I/R-induced injury was found in studies using the immunodeficient RAG−/− mice, which do not have any antibodies (10). These mice are highly resistant to intestinal I/R-induced injury in association with significantly reduced IL-6 production but become susceptible again after reconstitution with purified serum IgM from naïve WT mice, highlighting the critical role of natural IgM in the development of intestinal I/R-induced injury (10–14). Additionally, recent studies have demonstrated the initiation of intestinal I/R-induced injury is not an inherent property of all natural IgM but a subset of natural IgM termed pathogenic natural IgMs (14–17). Despite the established role of natural IgM in the pathogenesis of intestinal I/R-induced injury, regulatory mechanisms underlying the production of natural IgM are inadequately studied.
Natural IgM is spontaneously secreted by B1 B cells (18–20). B1 B cells can be further subdivided into B1a (CD5+) and B1b (CD5−) (21). Although still debatable, it has been demonstrated that B1a cells outside of the peritoneal cavity produce the majority of the natural IgM in naïve mice independent of T cells (18, 22, 23), whereas B1b cells are responsible for T-independent IgM memory response (24) but are not a major source of natural IgM. How these natural IgM-producing B1a cells are regulated are also poorly understood despite the findings that antigen specificity and B cell receptor (BCR) signaling strength are critical factors in B1a cell development because deletion of BCR co-stimulatory molecules such as CD19 results in a massive reduction of B1a numbers, whereas deletion of negative regulators of BCR signaling such as Siglec-G leads to a vast increase in B1a cell population (25, 26).
CD6 is a cell surface glycoprotein receptor originally discovered as a marker of T cells and was found present on a subset of human B cells (27, 28). The precise function of CD6 in T cells is still uncertain. Previous studies suggested that CD6 is a costimulatory molecule that can synergize with the T cell receptor to enhance and/or inhibit T cell activation (29–32). Compared with the undefined role of CD6 on T cells, its role in B cells is even less clear. There has been only one report providing in vitro evidence, suggesting that CD6 could regulate apoptosis of chronic lymphocytic leukemia B cells (33). The distribution of CD6 on murine B cells, whether it has any role in natural IgM production and in the development of intestinal I/R induced injury is completely unknown.
In this study, using WT and CD6−/− mice, we studied the potential role of CD6 in regulating intestinal I/R-induced injury by comparing mucosal histopathology, local IL-6 production, and serum IgM titers. We explored the underlying mechanism by examining the distribution, regulation, and effect of CD6 on B1a cells. Our results showed the first evidence that CD6 is expressed on mouse B1a B cells and that CD6 regulates intestinal I/R-induced injury by modulating natural IgM-producing B1a cell self-renewal.
Results
CD6−/− Mice Are Protected from Intestinal I/R-induced Injury
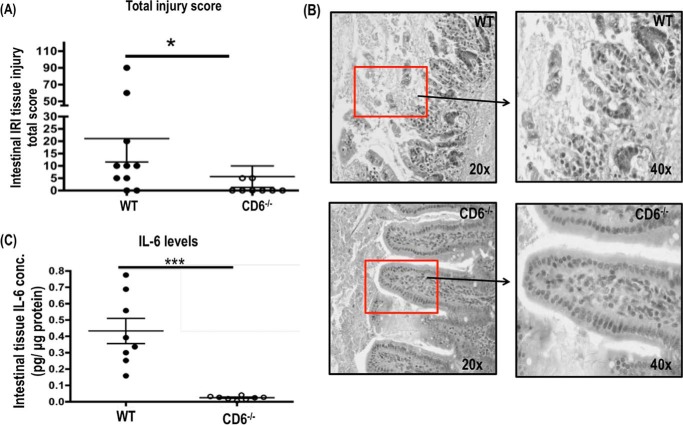
To explore whether CD6 has any role in the gut epithelial barrier breaching and mucosal damage after I/R, we induced intestinal I/R-induced injury in sex- and age-matched WT and CD6−/− mice following previously published protocol (10) and compared the clinical scores of the jejunum as well as local levels of IL-6 in these intestinal segments. We found that CD6−/− mice showed marked increased epithelial cell layer integrity within the intestinal villi (Fig. 1B) with 3.5-fold lower tissue damage in the intestine after I/R than WT as quantified by clinical scores assigned in a blinded fashion by a pathologist (Fig. 1A). In correlation with these histopathological analysis results in the CD6−/− mice after intestinal I/R, ELISA analyses of the jejunum tissue lysate also found 22-fold lower levels of IL-6 than those in WT mice (Fig. 1C). Thus, the severity of intestinal I/R-induced injury is significantly attenuated in the CD6−/− mice, demonstrating a previously unknown role of CD6 in this pathological condition.
FIGURE 1.
CD6−/− mice are protected from intestinal ischemia/reperfusion-induced injury. A, after intestinal ischemia reperfusion injury. The jejunum section of the intestine from WT and CD6−/− mice was prepared and stained with hematoxylin and eosin. Pathological scores of the intestinal injury were assigned in a masked fashion; n = 10. B, the representative pathologic features of injury are indicated by the squares. Magnification, 20× and 40× of tissue injury in the jejunum section of WT and CD6−/− mice. C, the local levels of IL-6 in the jejunum lysate were quantified by ELISA. Data are representative of n = 10 per group. *, p < 0.01; ***, p < 0.0001.
Pathogenic Natural IgMs Are Integrally Involved
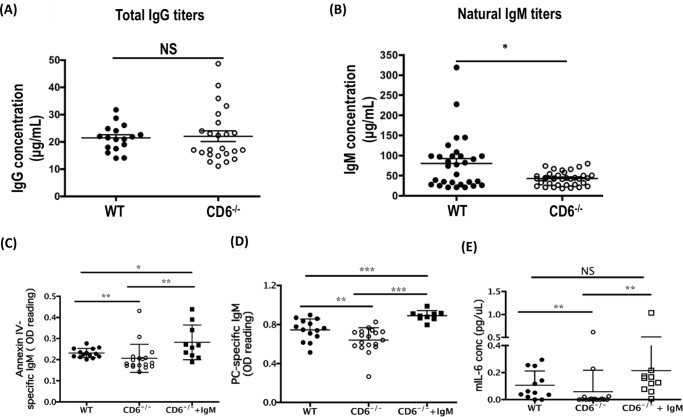
It has been previously reported that natural IgM plays a critical role in inducing mucosal damage in this model of intestinal I/R-induced injury (10). To understand the mechanism by which deficiency of CD6 protected mice from intestinal I/R-induced injury, we measured serum levels of total IgM in the serum of naïve WT and CD6−/− mice. We found that although the titers of total IgG in the serum were similar between WT and CD6−/− mice (Fig. 2A), the titers of natural IgM were reduced 1.9-fold in the CD6−/− mice compared with WT mice (Fig. 2B). Because natural IgM is reported to be polyreactive and only the pathogenic natural IgMs, e.g. anti-phosphorylcholine (PC) IgM and anti-annexin IV IgM, are critical in initiating intestinal I/R-induced injury (12, 34), we specifically measured titers of these pathogenic IgMs in the WT and CD6−/− mice that were used in the I/R studies and found a 1.2- and 1.4-fold reduction of annexin IV- and PC-specific IgM titers, respectively, in the sera of CD6−/− mice (Fig. 2, C and D). To determine that reduced titers of pathogenic natural IgMs protected CD6−/− mice from I/R-induced injury, we reconstituted IgM in the CD6−/− mice by tail vein i.v. injection of IgMs purified from naïve WT mice before I/R procedures. We found that reconstitution restored the titers of the PC and annexin V-reactive IgMs in the CD6−/− mice to those in the WT mice (Fig. 2, C and D) and that these reconstituted CD6−/− mice showed increased local inflammation after I/R (Fig. 2E).
FIGURE 2.
The titers of natural antibodies are reduced in the CD6−/− mice. Sera from naïve WT and CD6−/− mice were analyzed for total IgG (A) and total IgM (B) by ELISA. Data are representative of mIgM WT (n = 30), CD6−/− (n = 36), mIgG WT (n = 17), and CD6−/− (n = 24). Sera from WT, CD6−/−, and CD6−/− mice supplemented with WT IgM used in the intestinal I/R-induced injury studies were analyzed for annexin IV (C) and PC-specific IgM levels (D) by ELISA. Data are representative of WT (n = 14), CD6−/− (n = 18), and CD6−/− + IgM (n = 9). E, the local jejunum tissue lysates levels of IL-6 in the WT, CD6−/−, and CD6−/−+IgM after intestinal I/R-induced injury were quantified by ELISA. Data are representative of WT (n = 14), CD6−/− (n = 18), and CD6−/− + IgM (n = 9). *, p < 0.01; **, p < 0.001; ***, p < 0.0001. NS, not significant.
CD6 Is Selectively Expressed on B1 Cells outside the Bone Marrow and Peritoneal Cavity
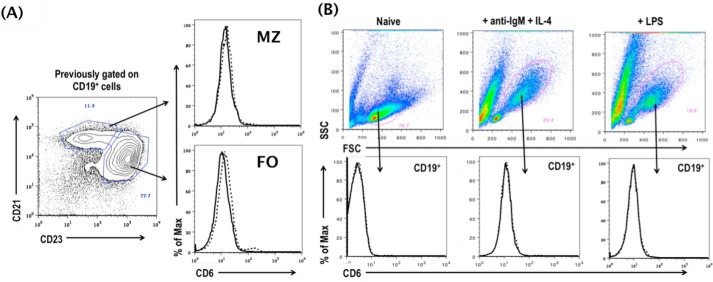
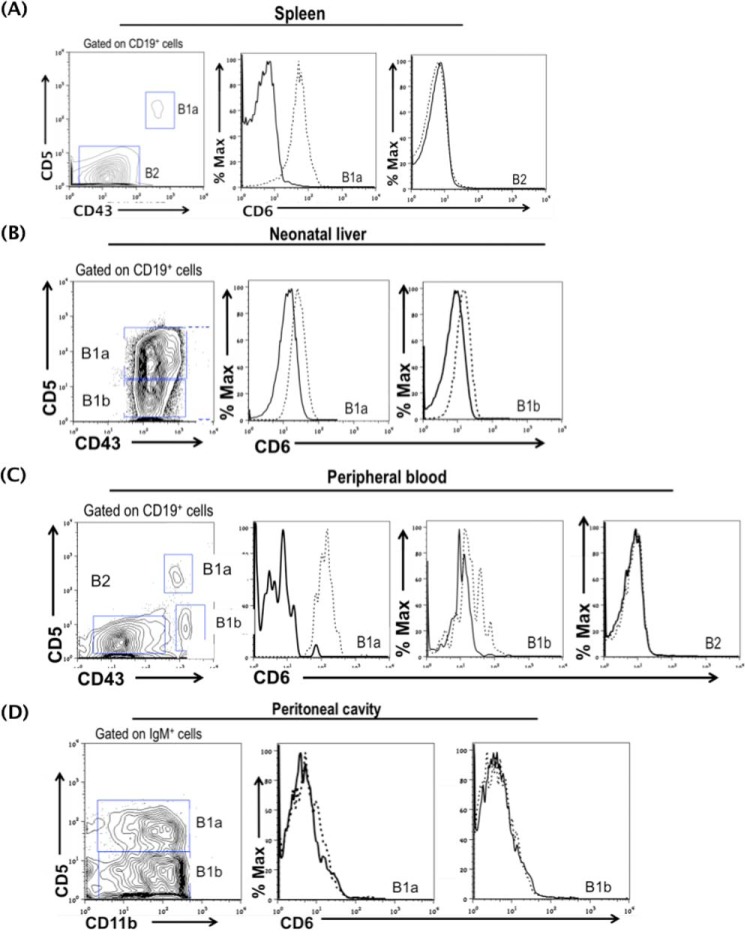
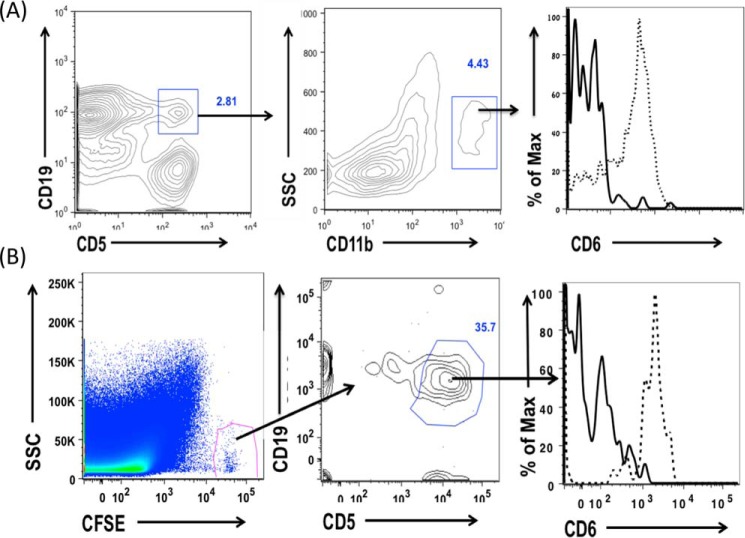
The above-described results suggest that CD6 could have a previously unknown role in regulating B1a cells, which are the source of natural IgM. To test this, we first examined the expression of CD6 on all B cells and then focused on the B1a cells. We found that CD6 is not expressed on splenic B2 cells (specifically marginal and follicular B cells) either constitutively (Fig. 3A) or after in vitro activation (Fig. 3B). Interestingly, using B1 cells from CD6−/− mice as controls, we found that CD6 is expressed on B1 cells in the spleen (Fig. 4A), neonatal liver (Fig. 4B), and peripheral blood (Fig. 4C) but not in the peritoneal cavity (Fig. 4D) and bone marrow (supplemental Fig. 2A). Among the B1 cell subsets in the spleen, neonatal liver, and peripheral blood, we found that the expression of CD6 was highly expressed on B1a cells compared with B1b cells. The full flow gating strategy for B1 B cells in the spleen, neonatal liver, peripheral blood, peritoneal cavity, and bone marrow are shown in supplemental Fig. 1, A–D, and Fig. 2B, respectively).
FIGURE 3.
CD6 is not detectable on resting or activated B2 cells. A, the expression of CD6 was analyzed on naive marginal (MZ; CD19+CD21+CD23−) and follicular (FO) B cells (CD19+CD21−CD23+) cells by flow cytometry. Data are representative of n = 8. CD6−/− mice were used as background FACS staining control. The solid line indicates CD6−/− mice, and the dashed line indicates CD6 expression on WT marginal and follicular B cells. B, splenocytes were isolated from WT and CD6−/− mice and stimulated in vitro with 10 μg/ml anti-IgM +IL4 (1000 units/ml) or 5 μg/ml LPS, and the expression of CD6 was analyzed on CD19+ cells 72 h post in vitro stimulation by flow cytometry. The solid line indicates CD6−/− mice, and the dashed line indicates CD6 expression on WT CD19+ cells. Data are representative of four experiments. SSC, side scatter.
FIGURE 4.
CD6 is expressed on non-peritoneal B1 cells. The expression of CD6 was analyzed on splenic B1a cells (A), neonatal liver B1a and B1b cells (B), peripheral blood B1a and B1b cells (C), and peritoneal B1a and B1b cells (D). CD6 expression was also analyzed on B2 cells in the spleen and peripheral blood. Splenic, neonatal, and peripheral blood B1a cells are CD19+CD43+CD5+, B1b cells are CD19+CD43+CD5−, and B2 cells are CD19+CD43−CD5−. Peritoneal B1a cells are IgM+CD11b+CD5+ and peritoneal B1b cells are IgM+CD11b+ CD5−. The solid line indicates CD6 staining on CD6−/− B1 cells, which were used as the FACS negative control, and the dotted line indicates CD6 staining on WT B1 cells. Data are representative of splenic B1 cells (n = 20), neonatal liver B1 cells (n = 3), peripheral blood (n = 4), and peritoneal cavity (n = 5).
Peritoneal B1a Cells Gain CD6 Expression outside the Peritoneal Cavity
Previous studies showed that B1a cells homeostatically migrate back and forth between the peritoneal cavity and the spleen (35, 36). To explore the mechanism by which CD6 is not present on B1a cells in the peritoneal cavity, we tested whether these B1a cells will gain the expression of CD6 after they move out the peritoneal cavity. To do this we first examined the expression of CD6 on “migrant” peritoneal B1a cells (CD19+CD5+CD11b+) and found that CD6 is expressed on these migrant peritoneal B1a cells in the spleen (Fig. 5A). To further confirm this result, we isolated peritoneal cells from WT mice, labeled them with carboxyfluorescein succinimidyl ester (CFSE), then injected them i.p. into naïve CD6−/− recipient mice. To induce the adoptively transferred B1a cells to migrate out of the peritoneal cavity, we injected the recipient mice with LPS i.p. immediately after (35). At 72 h, we analyzed CD6 expression on the CFSE+ peritoneal cells in the CD6−/− recipient mice and found that these adoptively transferred peritoneal B1a cells gained CD6 expression in the spleen (Fig. 5B), suggesting that CD6 expression is induced on peritoneal B1a cells after they exited the peritoneal environment.
FIGURE 5.
Peritoneal B1a cells gain CD6 expression outside the peritoneal cavity. A, splenocytes from WT and CD6−/− mice were isolated, and CD6 expression was analyzed on migrant peritoneal B1a cells in the spleen by gating on CD19+ CD5+ double-positive cells (B1a cells) and then assessing the expression of CD6 on B1a cells that are CD11b positive (migrant peritoneal B1a cells). Data are representative of 10 independent experiments. The solid line indicates CD6 background expression on migrant peritoneal B1a cells from CD6−/− mice, and the dotted line indicates CD6 expression on WT migrant peritoneal B1a cells. B, peritoneal cells from WT mice were isolated and labeled in vitro with CFSE. These labeled peritoneal cells were adoptively transferred into the peritoneal cavity of CD6−/− recipient mice. LPS was administered to these mice 2 h post-adoptive transfer, and CD6 expression was analyzed on migrated peritoneal B1a cells in the spleen 72 h later by gating on CFSE+, CD19+, and CD5+ cells. Data are representative of n = 4. The solid line indicates CD6 expression on CFSE-labeled peritoneal B1a cells from the CD6−/− mice that migrated to the spleen of CD6−/− mice, and dotted line indicates CD6 expression on CFSE-labeled WT peritoneal B1a cells that migrated to the spleen of CD6 −/− mice.
The B1 Cell Population Is Reduced in the Absence of CD6 in Select Tissue Compartments
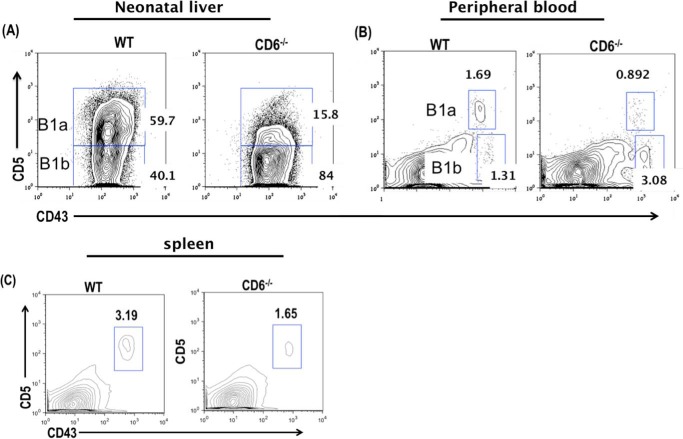
Our data so far showed that 1) lack of CD6 protected mice from intestinal I/R injury, in which natural IgM plays a critical role in the pathogenesis, 2) blood levels of natural IgM were significantly reduced in the CD6−/− mice, and 3) CD6 is expressed on B1 cells outside of the peritoneal cavity and bone marrow, which are one of the major sources of natural IgM. To determine why deficiency of CD6 leads to reduce IgM production, we compared the B1a cell population in the neonatal liver, peripheral blood, and spleen of WT and CD6−/− mice by flow cytometry. We found a 4- and 2.5-fold reduction in the B1a cells frequency and absolute numbers in the CD6−/− mice neonatal liver, respectively (Fig. 6A). The decrease of the B1a cell population in the CD6−/− neonatal liver was compensated with a 1.7- and 2.9-fold increase in the B1b cell frequency and absolute numbers as anticipated (Fig. 6A, Table 1). In the peripheral blood, the frequency of B1a cells was decreased by 1.5-fold in the CD6−/− mice (Fig. 6B, Table 1). The frequency of B1b cells in the peripheral blood was increased by 1.4-fold in the CD6−/−mice (Fig. 6B, Table 1).
FIGURE 6.
The B1a cell populations are reduced in the absence of CD6 outside the peritoneal cavity. A, flow cytometric analysis of day-7 old neonatal liver B1a cells by gating on CD19+CD43+CD5+ cells. Data are representative of n = 3 per group. B, flow cytometric analysis of peripheral blood B1a cells (CD19+CD43+CD5+). Data are representative of n = 4 mice per group. C, flow cytometric analysis of splenic B1a cells (CD19+CD43+CD5+). Data are representative of n = 11 mice per group. *, p < 0.01; **p < 0.001; ***, p < 0.0001.
TABLE 1.
Frequency and absolute numbers of B1 cells for WT and CD6−/− mice
Data are mean values ± S.D.
| B1 cell location | Mice (n) | Frequency |
|
|---|---|---|---|
| WT | CD6−/− | ||
| % | |||
| Neonatal liver B1a cells | 47 ± 12 | 12 ± 2a | |
| Neonatal liver B1b cells | 3 | 50.33 ± 6.810 | 87 ± 1.06a |
| Peripheral blood B1a cells | 4 | 2.41 ± 0.4035 | 1.635 ± 0.267 |
| Peripheral blood B1b cells | 4 | 2.010 ± 0.459 | 2.873 ± 0.158 |
| Splenic B1a cells | 11 | 1.4 ± 0.3 | 0.7 ± 0.8a |
| B1 cell location | Mice (n) | Absolute cell numbers |
|
|---|---|---|---|
| WT | CD6−/− | ||
| Neonatal liver B1a cells | 3 | 3,253,000 ± 655,500 | 1,277,000 ± 99,390a |
| Neonatal liver B1b cells | 3 | 3,327,000 ± 361,200 | 9,530,000 ± 240,100b |
| Peripheral blood B1a cells | 4 | N/A | N/A |
| Peripheral blood B1b cells | 4 | N/A | N/A |
| Splenic B1a cells | 11 | 403,800 ± 34,760 | 160,900 ± 26,750a |
a p < 0.001.
b p < 0.0001.
In the spleen we found a 2- and 2.5-fold decrease in the frequency and absolute numbers of B1a cells in the CD6−/−mice (Fig. 6C, Table 1). In the bone marrow, we found no significant difference in the frequency and absolute numbers of B1 cells (supplemental Fig. 2C and Table 2). In the peritoneal cavity we made some interesting observations. We found a 1.2- and 1.6-fold reduction in the B1 cell frequency and absolute numbers,+ respectively, in the CD6−/− mice. Upon further analysis of the B1 cell subsets, we found a 1.8-fold increase in the B1a cell frequency in the CD6−/− mice. Although there was a 1.3-fold increase in the total B1a cell numbers in the CD6−/−, there was no significant difference compared with WT mice. Additionally, in the CD6−/− peritoneal cavity we found a 1.2- and 1.7-fold compensatory reduction in the B1b cell frequency and absolute numbers, respectively (supplemental Fig. 2D and Table 2). Together, these data indicate that CD6 is required to maintain a normal B1a cell population in these tissues, and the significantly reduced population of B1a cells in CD6−/− mice could explain the markedly reduced blood IgM levels that we found in the above experiments.
Reduced B1a Cell Population in CD6−/− Mice Is Due to Impaired B1a Cell Self-renewal
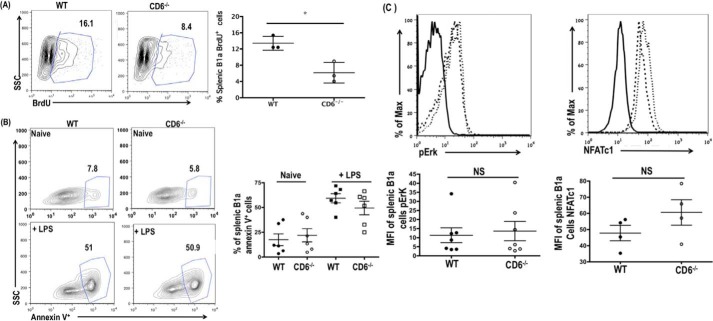
We then explored the possible mechanism by which the absence of CD6 resulted in the reduced numbers of B1a cells in the CD6−/− mice. The B1a cell population is maintained by their unique ability to undergo self-renewal (37, 38). In asplenic mice, the B1a cell population is significantly reduced in multiple tissue compartments, highlighting the importance of the spleen in B1a self-renewal (39). To determine if the reduced B1a cell population in the CD6−/− mice is caused by their impaired ability to undergo self-renewal, we measured B1a cell proliferation between WT and CD6−/− mice using a BrdU incorporation assay. Incorporation of BrdU by splenic B1a cells was reduced by 1.5-fold in the CD6−/− mice (Fig. 7A). To determine if CD6 plays an intrinsic role in B1a cell proliferation, we also assessed BrdU incorporation of peritoneal B1a and bone marrow B1 cells that do not express CD6. We found no significant difference in either peritoneal B1a (supplemental Fig. 3A) or bone marrow B1 cell proliferation between WT and CD6−/−mice (supplemental Fig. 3B). In addition to impaired self-renewal, reduced B1a cell population in CD6−/− mice may result from enhanced apoptosis of these cells given that CD6 has been implicated in the survival of B1a-derived B-CLL cells (33). We compared the frequency of apoptotic splenic B1a cells in WT and CD6−/− mice before and after LPS stimulation by annexin V staining. There were no differences in splenic B1a apoptosis basally or upon activation (Fig. 7B). These data indicate that the reduction of B1a cell population in the CD6−/− mice is due to impaired self-renewal of B1a cells and not enhanced apoptosis.
FIGURE 7.
Reduced B1a cell population in CD6−/− mice is due to impaired B1a cell self-renewal. A, WT and CD6−/− splenic B1a cell incorporation of BrdU (CD19+CD43+CD5+BrdU+) were analyzed by flow cytometry. Data are representative of n = 3 mice per group. B, the frequency of annexin V+ splenic B1a cells (CD19+CD43+CD5+annexin V+) were analyzed basally and upon LPS (5 μg/ml) stimulation for 24 h. Data are representative of n = 6. C, WT and CD6−/− splenic B1a pErk and NFATc1 expression levels were analyzed by flow cytometry. The quantification of pErk and NFATc1 is shown in the lower panel. Data are representative of seven and four independent experiments (pERK and NFATc1, respectively). The solid dark line indicates isotype control, the dashed dark line indicates WT, and the thin dotted line indicates CD6−/−. *, p < 0.01; NS, not significant.
Elevated basal Erk phosphorylation is one of the hallmark features of naive B1a cells (40). To determine whether the reduced splenic B1a cell self-renewal in CD6−/− mice is associated with impaired Erk phosphorylation, we compared the basal Erk phosphorylation of WT and CD6−/− splenic B1a cells. CD6−/− splenic B1a cells displayed a slight increase in basal Erk phosphorylation compared with WT; however, the difference was not significant (Fig. 7C). We also analyzed the expression levels of NFATc1, a transcription factor that has been shown to be important for B1a cell development/survival (41). A slightly elevated but insignificant increase in NFATc1 expression levels was detected in CD6−/− splenic B1a cells (Fig. 7D). Taken together, these data suggest that reduced self-renewal of CD6-deficient B1a cells is independent of impaired Erk signaling and NFATc1 activity.
Discussion
In this report we studied CD6−/− mice in a model of intestinal I/R-induced injury, a devastating and likely lethal pathological condition. We found that CD6−/− mice were protected from intestinal I/R-induced injury as shown by significantly attenuated histopathology of the intestine and reduced levels of local IL-6. In mechanistic studies we found that total annexin IV, and PC-specific IgM titers were reduced in the CD6−/− mice and that reconstitution of CD6−/− mice with IgM from naïve WT mice significantly restored intestinal I/R-induced inflammation. In addition, we demonstrated that CD6 was selectively expressed on natural antibody producing-B1 cell outside of the peritoneal cavity and bone marrow. Finally, we found that CD6-expressing B1 cells were significantly reduced in select tissue compartments in the CD6−/− mice in association with reduced B1a cell proliferation.
Intestinal I/R-induced injury is a condition frequently associated with high morbidity and mortality in patients with trauma and surgeries, especially small intestine transplantation (1). The unexpected results that CD6−/− mice were protected in the intestinal I/R-mediated injury demonstrated a previously unknown role of CD6 in the pathogenesis of this condition. CD6 was originally identified as a marker for T cells and has been associated with multiple autoimmune diseases including rheumatoid arthritis and psoriasis (42, 43). Quantitative proteomics analysis of signalosome dynamics in T cells identifies CD6 as a Lat adaptor-independent TCR signaling hub, suggesting that CD6 modulates TCR signaling (44). A recent study using CD6-deficient mice found exacerbated collagen-induced arthritis in the CD6−/− mice (45), a surprising result because anti-CD6 mAbs showed efficacy in treating patients with rheumatoid arthritis (46) and psoriasis (47). Compared with the debatable role of CD6 on T cells, information on the potential role of CD6 on B cells is even more limited. So far, it has only been reported that a proportion of blood CD6+ B cells is reduced in primary Sjögren's syndrome patients (28) and that CD6 ligation modulates the Bcl-2/Bax ratio and protects chronic lymphocytic leukemia B cells from apoptosis (49). Our results that CD6 deficiency led to impaired B1a cell self-renewal, reduced B1a cell population, and natural IgM titers in the blood showed a new role for CD6 in regulating B cells for the first time.
The discovery that CD6−/− mice have reduced titers of pathogenic natural IgM and are protected from intestinal I/R-induced injury is consistent with previous reports that natural IgM is critical in I/R-induced injury (10, 13). RAG1−/− mice, which do not have any antibodies, are highly resistant to intestinal I/R-induced injury but become susceptible again after reconstitution with purified serum IgM from naïve WT mice or an IgM mAb alone (clone B4), which specifically recognizes annexin IV (15). In addition to annexin IV, natural IgM reactive to PC has also been implicated in the pathogenesis of intestinal I/R-induced injury (12, 15, 50). Consistent with these previous reports, we found that natural IgM specific for PC and annexin IV were significantly reduced in the blood of CD6−/− mice compared with WT mice in association with decreased intestinal I/R-induced injury and that supplementing IgMs isolated from naïve WT mice into CD6−/− mice increased titers of both PC- and annexin IV-specific IgMs, leading to exacerbated intestinal inflammation after I/R.
Beside nature IgM, several studies have implicated a role for T cells in this acute inflammatory model (51–53). The recent report of impaired Treg function in the CD6−/− mice along with enhanced susceptibility to a model of T cell-mediated autoimmune disease would suggest that CD6 deficiency should result in exacerbated intestinal I/R-induced injury if CD6-mediated function on T cell is critical in this acute inflammatory model. However, the finding that CD6−/− mice are protected from intestinal I/R-induced injury in association with reduced natural IgMs and that reconstitution with IgM in CD6−/− mice restored their susceptibility to intestinal I/R-induced injury emphasizes the significant contribution of CD6-mediated function on natural antibody secreting B1a cells in the pathogenesis.
The absence of CD6 expression on mature B2 cells in mice was an interesting finding because CD6 is reported to be present on mature B2 cells in humans (28). CD6 is closely related to the lymphocyte receptor CD5 (54). These two genes are immediately adjacent to each other and are highly homologous members of the group B scavenger receptor cysteine-rich (SRCR) superfamily of protein receptors, previously reported to be co-expressed on the same cells (54, 55). In mouse B cells, CD5 is not expressed on naïve B2 cells; however anti-IgM stimulation of B2 cells induces CD5 expression (56). In our studies, in vitro stimulation of mouse B2 cells did not induce appreciable expression of CD6. These findings suggest that although CD5 and CD6 are closely related, the mechanism regulating their expression in mouse B cells is distinct and different from T cells wherein expression of CD5 and CD6 appears coordinated.
The discovery that CD6 is differentially expressed on mouse B1 cells adds CD6 to a list of unique markers that are differentially expressed between peritoneal and splenic B1 cells (57). This finding also indicates that bone marrow B1 and splenic B1 cells may also express differential markers. Differential expression of CD6 raises the possibilities that CD6+ B1 and CD6− B1 cells may arise from two distinct B1 cell lineages and/or that CD6 expression is heavily influenced by the local microenvironment. The expression of CD6 on migrant peritoneal B1a cells in the spleen and the discovery that WT peritoneal B1a cells acquire CD6 expression in the spleen of CD6−/− recipient mice after adoptive-transfer strongly supports the notion that the microenvironment greatly influences expression of CD6 and drives phenotypic differences in B1a cells (58, 59).
The deletion of certain BCR signaling positive regulators results in the massive reduction of B1a cells (37). Reduction of the B1a cell population outside the bone marrow and peritoneal cavity in the CD6−/− mice suggest that CD6 is a positive regulator of BCR signaling. In T cells, CD6 can initiate its own signaling hub and tune overall T cell receptor signaling threshold thru its ability to recruit signaling molecules such as SLP-76 and Vav1 (44). Given that enhanced BCR signaling is one of the critical factors in B1a development, our data support the hypothesis that CD6 signaling contributes to the overall BCR signaling threshold that shapes the B1a development process.
The B1 cells in the bone marrow and peritoneal cavity do not express CD6. As anticipated, the deficiency of CD6 did not alter the B1 cell population in the bone marrow. In contrast, we found reduced B1 cells in the peritoneal cavity of CD6−/− mice. These findings are consistent with the recent study indicating that serum IgM-deficient mice display reduced B1 cell population in the peritoneal cavity and normal B1 cell population in the bone marrow (48). These serum IgM-deficient mice also harbor large amounts of anergic CD5+ B cells, which potentially could account for the elevated CD5+ B1a cells population in the peritoneal cavity of CD6−/− mice. The discovery that the B1 cell population in the CD6−/− mice bone marrow are not altered despite altered B1 cell population in the peripheral tissue suggest a potential defect in the renewal/proliferation rather than in the development of B1 cells. We assessed the proliferation of B1a cells and discovered that CD6-deficient mice displayed reduced proliferation compared with WT. Specifically, the defect was only found in CD6-expressing B1a cells. These data suggest that CD6 plays an intrinsic role in B1a cell self-renewal.
Thus, our working model is that CD6 deficiency impairs the self-renewal of natural IgM-secreting B1a cells, therefore reducing the B1a cell population. The reduction of the B1a cell population results in reduced pathogenic natural antibodies (annexin IV and PC-specific IgM) in the blood. Reduced recognition of ischemic neo-antigens by natural IgM subsets in the CD6−/− mice led to impaired inflammatory responses such as IL-6 production during reperfusion of the intestine, and, consequently, attenuated mucosal damage. This study provides new insight into the role of CD6 in the inflammatory process that induces intestinal I/R-induced injury and on B1a cells that produce natural IgM, which drives the onset of intestinal I/R-induced injury.
Materials and Methods
Mice
CD6−/− mice were developed by manipulating ES cells using a conventional homologous recombination strategy and blastocyst injection of the identified target ES cells. The resultant CD6−/− mice were bred onto the DBA/1 background by >12 generations of backcrossing. PCR and Southern blot assays verified the deletion of the targeted loci, and flow cytometry analysis of CD6 protein on T cells confirmed the deficiency of CD6 in the CD6−/− mice (details will be described in another report). All mice were maintained in the animal facility at the Cleveland Clinic, and all animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Mouse Intestinal I/R Model
The intestine ischemia and reperfusion was performed on mice at 8–12 weeks of age. Surgical instruments were sterilized by autoclave before surgery. The mice were anesthetized with 4% isoflurane inhalation for induction and 0.5% for maintenance of anesthesia using an approved anesthetic gas machine. The mouse abdomen skin was cleaned and entered via a midline laparotomy incision. The superior mesenteric artery was identified and isolated, and then the VASCU-STATT Plus bulldog clamp (Scanlan, Saint Paul, MN) was utilized to perform the vascular exclusion for 30 min. A heating pad was applied to keep the mouse body temperature around 37 °C. After 30 min of mesenteric ischemia phase, the clamp was removed, and the midline laparotomy incisions were closed with 4–0 silk suture; the intestine was reperfused for 3 h before the tissue (jejunum) was harvested. For some experiments, CD6−/− mice were reconstituted with 100 μg of mouse IgM (Rockland Immunochemicals Inc., Limerick, PA) mouse intravenously 30 min before the start of the intestinal ischemia-surgery.
Local IL-6 Assay
The jejunum tissue was mechanically homogenized in Nonidet P-40 lysis buffer (Invitrogen) containing a tablet of protease inhibitors (Roche Applied Science). The homogenized tissue was incubated on ice for 30 min with brief vortexing every 5 min. Tissue lysates were centrifuged at 13,000 rpm for 30 min at 4 °C, the pellets were discarded, and protein concentration of the supernatant was measured using the Pierce BCA Protein Assay kit. For the detection of IL-6 levels in the supernatant, 96-well high binding plates were coated with 2 μg/ml rat anti-mouse IL-6 (BioLegend) diluted in 1× PBS. Protein lysates were diluted 1/20 and loaded onto the coated ELISA plate. Bound IL-6 was detection using biotin anti-mouse IL-6 (BioLegend) and subsequently using avidin-HRP (BioLegend). The ELISA color reaction was initiated using tetramethylbenzidine (TMB) substrate (Thermo Scientific). 2 m H2SO4 was used to stop the TMB reaction, and absorbance at 450 nm was measured. IL-6 concentration was normalized to the starting initial protein concentration.
Histology and Injury Scoring
Histopathological examination and tissue injury score was performed in a blinded manner by a pathologist on 4-μm-thick paraffin-embedded sections stained with hematoxylin and eosin. Digital images were captured with a Leica-DM5500 B microscope camera (Buffalo Grove, IL) and analyzed with Image-Pro software (Media Cybernectics, Silver Spring, MD).
Flow Cytometry
Single-cell suspensions of spleen, peritoneal lavage from adult mice, or day-7 old neonatal liver were incubated for 10 min on ice with anti-mouse CD16/32 Fc blocking antibody (BioLegend). After Fc blocking, cells were incubated with varying combinations of the following antibodies: anti-CD19, anti-CD43, anti-CD5, anti-CD6 clone 34 (prepared in the laboratory), anti-CD11b, anti-CD80, anti-CD86, anti-IgM, anti-CD23, and anti-CD21. All antibodies were purchased from BioLegend. Cells were stained in FACS buffer, and expression of cell surface markers was acquired on both BD LSRFortessa and FACSCalibur flow cytometer. Acquired flow cytometer data were analyzed using the Flowjo software. For splenic B1a intracellular staining, splenocytes from WT and CD6−/− mice were first incubated with antibodies to CD19, CD43, and CD5 as previously described. After cell surface staining, the cells were fixed and permeabilized using the BD Biosciences permeabilization kit according to the manufacturer's instruction. The permeabilized splenocytes were incubated with anti-pERK (T204/Y202) (eBioscience), NFATc1 (BioLegend), mouse IgG1 isotype control (BD Bioscience), or rat isotype control antibody (BioLegend).
Splenic B2 Cells in Vitro Stimulation
Single-cell suspensions of spleen were plated at a density of 106 cells/ml in a 96-well plate. For the detection of CD6 expression on splenic B2 cells, splenocytes from WT and CD6−/− mice were harvested after 72 h in vitro stimulation with 10 μg/ml goat F(ab′)2 anti-IgM and IL-4 (1000 units/ml) (Peprotech) or 5 μg/ml of LPS (Sigma), and CD6 expression was analyzed on activated CD19+ (B2 cells) by flow cytometry.
Peritoneal B1a Cells Migration to the Spleen
Peritoneal cells were isolated from WT and CD6−/− mice by peritoneal lavage. 5 × 106 cells were labeled in vitro with CFSE (Invitrogen) and transferred into the peritoneal cavity of CD6−/− recipient mice. Two hours post-adoptive transfer, 5 μg of LPS (Sigma) was also administered into the peritoneal cavity. After 72 h, splenocytes were isolated, and CD6 expression was analyzed on CFSE-positive cells in addition to gating on CD19 and CD5 double-positive cells (CFSE+CD19+CD5+).
BrdU Incorporation
WT and CD6−/− mice were intraperitoneally injected with 500 μl of BrdU (BD Biosciences) at a diluted concentration of 1 mg/ml every other day for 7 days. At the end of the study, splenocytes, bone marrow, and peritoneal cavity cells were isolated and stained with various antibodies (CD19, CD43, and CD5; CD19 and CD43; B220, CD11b, and CD5), respectively. After cell surface staining on ice, the cells were fixed and permeabilized. After permeabilization, the cells were intracellular stained with FITC-labeled anti-BrdU antibody (BD Biosciences) and analyzed by flow cytometry.
ELISA
For the detection of total IgG or IgM serum levels, 96-well high binding plates (Greiner Bio-One) were coated with 0.34 μg/ml rabbit anti-mouse IgM+IgG (H+L) (Jackson ImmunoResearch). Serum samples were diluted 1/1000. Bound antibodies were detected using either goat anti-mouse IgM HRP (Southern Biotech) or rat anti-mouse IgG-HRP (Southern Biotech). For the detection of PC-specific IgM, plates were coated with 10 μg/ml PC-BSA (Biosearch Technologies). Serum samples were also diluted 1/1000, and bound PC-specific IgM antibodies were detected using goat anti-mouse IgM-HRP. For the detection of annexin IV-specific IgM, plates were coated with 1 μg/ml recombinant mouse annexin IV (LSBio). Serum samples were diluted 1/50, and bound annexin IV-specific IgM was detected using goat anti-mouse IgM-HRP. The ELISA color reaction was initiated using TMB substrate (Thermo Scientific, MA). 2 m H2SO4 was used to stop the TMB reaction, and absorbance at 450 nm was measured.
Statistical Analysis
Data were compared by unpaired t test or paired t test using the GraphPad Prism software with a two-tailed Student's t test of equal variance. The difference between the groups was considered significant at p < 0.05.
Author Contributions
G. E.-A., Y. L., and W. X. performed the experiments and analyzed the data. N. G. S. developed the mice on a Dba/1 background. N. G. S., N. G., and J. F. analyzed the data and edited the manuscript. F. L. supervised the study, analyzed the data, and wrote the manuscript together with G. E.-A. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
This work was supported by National Institutes of Health Grants NS081443 and AR061564 (to F. L.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Table 1 and Figs. 1–3.
- I/R
- ischemia/reperfusion
- BCR
- B cell receptor
- PC
- phosphorylcholine
- BrdU
- bromodeoxyuridine.
References
- 1. Lenaerts K., Ceulemans L. J., Hundscheid I. H., Grootjans J., Dejong C. H., and Olde Damink S. W. (2013) New insights in intestinal ischemia-reperfusion injury: implications for intestinal transplantation. Curr. Opin. Organ Transplant. 18, 298–303 [DOI] [PubMed] [Google Scholar]
- 2. Pierro A., and Eaton S. (2004) Intestinal ischemia reperfusion injury and multisystem organ failure. Semin. Pediatr. Surg. 13, 11–17 [DOI] [PubMed] [Google Scholar]
- 3. Vollmar B., and Menger M. D. (2011) Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch. Surg. 396, 13–29 [DOI] [PubMed] [Google Scholar]
- 4. Grootjans J., Lenaerts K., Derikx J. P., Matthijsen R. A., de Bruïne A. P., van Bijnen A. A., van Dam R. M., Dejong C. H., and Buurman W. A. (2010) Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am. J. Pathol. 176, 2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braun F., Hosseini M., Wieland E., Sattler B., Müller A. R., Fändrich F., Kremer B., and Ringe B. (2004) Kinetics and localization of interleukin-2, interleukin-6, heat shock protein 70, and interferon γ during intestinal-rerfusion injury. Transplant. Proc. 36, 267–269 [DOI] [PubMed] [Google Scholar]
- 6. Ban K., and Kozar R. A. (2012) Protective role of p70S6K in intestinal ischemia/reperfusion injury in mice. PLoS ONE 7, e41584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuzzocrea S., De Sarro G., Costantino G., Ciliberto G., Mazzon E., De Sarro A., and Caputi A. P. (1999) IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. J. Leukoc. Biol. 66, 471–480 [DOI] [PubMed] [Google Scholar]
- 8. Tuboly E., Futakuchi M., Varga G., Érces D., To"okés T., Mészáros A., Kaszaki J., Suzui M., Imai M., Okada A., Okada N., Boros M., and Okada H. (2016) C5a inhibitor protects against ischemia/reperfusion injury in rat small intestine. Microbiol. Immunol. 60, 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu D. Z., Zaets S. B., Chen R., Lu Q., Rajan H., Yang X., Zhang J., Feketova E., Bogdan N., Deitch E. A., and Cao Y. (2009) Elimination of C5aR prevents intestinal mucosal damage and attenuates neutrophil infiltration in local and remote organs. Shock 31, 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams J. P., Pechet T. T., Weiser M. R., Reid R., Kobzik L., Moore F. D. Jr, Carroll M. C., and Hechtman H. B. (1999) Intestinal reperfusion injury is mediated by IgM and complement. J. Appl. Physiol. 86, 938–942 [DOI] [PubMed] [Google Scholar]
- 11. Austen W. G. Jr, Kobzik L., Carroll M. C., Hechtman H. B., and Moore F. D. Jr. (2003) The role of complement and natural antibody in intestinal ischemia-reperfusion injury. Int. J. Immunopathol. Pharmacol. 16, 1–8 [DOI] [PubMed] [Google Scholar]
- 12. Padilla N. D., van Vliet A. K., Schoots I. G., Valls Seron M., Maas M. A., Peltenburg E. E., de Vries A., Niessen H. W., Hack C. E., and van Gulik T. M. (2007) C-reactive protein and natural IgM antibodies are activators of complement in a rat model of intestinal ischemia and reperfusion. Surgery 142, 722–733 [DOI] [PubMed] [Google Scholar]
- 13. Zhang M., Alicot E. M., and Carroll M. C. (2008) Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Mol. Immunol. 45, 4036–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang M., Austen W. G. Jr, Chiu I., Alicot E. M., Hung R., Ma M., Verna N., Xu M., Hechtman H. B., Moore F. D. Jr, and Carroll M. C. (2004) Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 101, 3886–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulik L., Fleming S. D., Moratz C., Reuter J. W., Novikov A., Chen K., Andrews K. A., Markaryan A., Quigg R. J., Silverman G. J., Tsokos G. C., and Holers V. M. (2009) Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J. Immunol. 182, 5363–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleming S. D., Shea-Donohue T., Guthridge J. M., Kulik L., Waldschmidt T. J., Gipson M. G., Tsokos G. C., and Holers V. M. (2002) Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J. Immunol. 169, 2126–2133 [DOI] [PubMed] [Google Scholar]
- 17. Reid R. R., Woodcock S., Shimabukuro-Vornhagen A., Austen W. G. Jr, Kobzik L., Zhang M., Hechtman H. B., Moore F. D. Jr, and Carroll M. C. (2002) Functional activity of natural antibody is altered in Cr2-deficient mice. J. Immunol. 169, 5433–5440 [DOI] [PubMed] [Google Scholar]
- 18. Holodick N. E., Tumang J. R., and Rothstein T. L. (2010) Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur. J. Immunol. 40, 3007–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyaw T., Tay C., Krishnamurthi S., Kanellakis P., Agrotis A., Tipping P., Bobik A., and Toh B. H. (2011) B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 109, 830–840 [DOI] [PubMed] [Google Scholar]
- 20. Merino M. C., and Gruppi A. (2006) Origin and development of B1 lymphocytes: a cell population involved in defense and autoimmunity. Medicina 66, 165–172 [PubMed] [Google Scholar]
- 21. Stall A. M., Adams S., Herzenberg L. A., and Kantor A. B. (1992) Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann. N.Y. Acad. Sci. 651, 33–43 [DOI] [PubMed] [Google Scholar]
- 22. Choi Y. S., Dieter J. A., Rothaeusler K., Luo Z., and Baumgarth N. (2012) B-1 cells in the bone marrow are a significant source of natural IgM. Eur. J. Immunol. 42, 120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holodick N. E., Vizconde T., and Rothstein T. L. (2014) Splenic B-1a cells expressing CD138 spontaneously secrete large amounts of immunoglobulin in naive mice. Front. Immunol. 5, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehrenstein M. R., and Notley C. A. (2010) The importance of natural IgM: scavenger, protector. and regulator. Nat. Rev. Immunol. 10, 778–786 [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann A., Kerr S., Jellusova J., Zhang J., Weisel F., Wellmann U., Winkler T. H., Kneitz B., Crocker P. R., and Nitschke L. (2007) Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat. Immunol. 8, 695–704 [DOI] [PubMed] [Google Scholar]
- 26. Krop I., de Fougerolles A. R., Hardy R. R., Allison M., Schlissel M. S., and Fearon D. T. (1996) Self-renewal of B-1 lymphocytes is dependent on CD19. Eur. J. Immunol. 26, 238–242 [DOI] [PubMed] [Google Scholar]
- 27. Pinto M., and Carmo A. M. (2013) CD6 as a therapeutic target in autoimmune diseases: successes and challenges. BioDrugs 27, 191–202 [DOI] [PubMed] [Google Scholar]
- 28. Alonso R., Buors C., Le Dantec C., Hillion S., Pers J. O., Saraux A., Montero E., Marianowski R., Loisel S., Devauchelle V., Youinou P., and Renaudineau Y. (2010) Aberrant expression of CD6 on B-cell subsets from patients with Sjogren's syndrome. J. Autoimmun. 35, 336–341 [DOI] [PubMed] [Google Scholar]
- 29. Gimferrer I., Calvo M., Mittelbrunn M., Farnós M., Sarrias M. R., Enrich C., Vives J., Sánchez-Madrid F., and Lozano F. (2004) Relevance of CD6-mediated interactions in T cell activation and proliferation. J. Immunol. 173, 2262–2270 [DOI] [PubMed] [Google Scholar]
- 30. Nair P., Melarkode R., Rajkumar D., and Montero E. (2010) CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leukocyte cell adhesion molecule interaction. Clin. Exp. Immunol. 162, 116–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliveira M. I., Gonçalves C. M., Pinto M., Fabre S., Santos A. M., Lee S. F., Castro M. A., Nunes R. J., Barbosa R. R., Parnes J. R., Yu C., Davis S. J., Moreira A., Bismuth G., and Carmo A. M. (2012) CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur. J. Immunol. 42, 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zimmerman A. W., Joosten B., Torensma R., Parnes J. R., van Leeuwen F. N., and Figdor C. G. (2006) Long-term engagement of CD6 andALCAM is essential for T-cell proliferation induced by dendritic cells. Blood 107, 3212–3220 [DOI] [PubMed] [Google Scholar]
- 33. Osorio L. M., De Santiago A., Aguilar-Santelises M., Mellstedt H., and Jondal M. (1997) CD6 ligation modulates the Bcl-2/Bax ratio and protects chronic lymphocytic leukemia B cells from apoptosis induced by anti-IgM. Blood 89, 2833–2841 [PubMed] [Google Scholar]
- 34. Ansel K. M., Harris R. B., and Cyster J. G. (2002) CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16, 67–76 [DOI] [PubMed] [Google Scholar]
- 35. Yang Y., Tung J. W., Ghosn E. E., Herzenberg L. A., and Herzenberg L. A. (2007) Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. U.S.A. 104, 4542–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stoermann B., Kretschmer K., Düber S., and Weiss S. (2007) B-1a cells are imprinted by the microenvironment in spleen and peritoneum. Eur. J. Immunol. 37, 1613–1620 [DOI] [PubMed] [Google Scholar]
- 37. Baumgarth N. (2011) The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46 [DOI] [PubMed] [Google Scholar]
- 38. Hayakawa K., Hardy R. R., Herzenberg L. A., and Herzenberg L. A. (1985) Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161, 1554–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wardemann H., Boehm T., Dear N., and Carsetti R. (2002) B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong S. C., Chew W. K., Tan J. E., Melendez A. J., Francis F., and Lam K. P. (2002) Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. J. Biol. Chem. 277, 30707–30715 [DOI] [PubMed] [Google Scholar]
- 41. Berland R., and Wortis H. H. (2003) Normal B-1a cell development requires B cell-intrinsic NFATc1 activity. Proc. Natl. Acad. Sci. U.S.A. 100, 13459–13464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodriguez P. C., Torres-Moya R., Reyes G., Molinero C., Prada D., Lopez A. M., Hernandez I. M., Hernandez M. V., Martinez J. P., Hernandez X., Casaco A., Ramos M., Avila Y., Barrese Y., Montero E., and Hernandez P. (2012) A clinical exploratory study with itolizumab, an anti-CD6 monoclonal antibody, in patients with rheumatoid arthritis. Results Immunol. 2, 204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krupashankar D. S., Dogra S., Kura M., Saraswat A., Budamakuntla L., Sumathy T. K., Shah R., Gopal M. G., Narayana Rao T., Srinivas C. R., Bhat R., Shetty N., Manmohan G., Sai Krishna K., et al. (2014) Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J. Am. Acad. Dermatol. 71, 484–492 [DOI] [PubMed] [Google Scholar]
- 44. Roncagalli R., Hauri S., Fiore F., Liang Y., Chen Z., Sansoni A., Kanduri K., Joly R., Malzac A., Lähdesmäki H., Lahesmaa R., Yamasaki S., Saito T., Malissen M., Aebersold R., Gstaiger M., and Malissen B. (2014) Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor-independent TCR signaling hub. Nat. Immunol. 15, 384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Orta-Mascaró M., Consuegra-Fernández M., Carreras E., Roncagalli R., Carreras-Sureda A., Alvarez P., Girard L., Simões I., Martínez-Florensa M., Aranda F., Merino R., Martínez V. G., Vicente R., Merino J., et al. (2016) CD6 modulates thymocyte selection and peripheral T cell homeostasis. J. Exp. Med. 213, 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chopra A., Chandrashekara S., Iyer R., Rajasekhar L., Shetty N., Veeravalli S. M., Ghosh A., Merchant M., Oak J., Londhey V., Barve A., Ramakrishnan M. S., and Montero E. (2016) Itolizumab in combination with methotrexate modulates active rheumatoid arthritis: safety and efficacy from a phase 2, randomized, open-label, parallel-group, dose-ranging study. Clin. Rheumatol. 35, 1059–1064 [DOI] [PubMed] [Google Scholar]
- 47. Dogra S., D. S. K., Budamakuntla L., Srinivas C. R., Khopkar U., Gupta S., Shetty N., Pratap D. V., Gopal M. G., Rao T. N., Garg V., Sumathy T. K., Saraswat A., Bhat R., Kura M., Pandey N., et al. (2015) Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: A double-blind, randomized-withdrawal, placebo-controlled study. J. Am. Acad. Dermatol. 73, 331–333 [DOI] [PubMed] [Google Scholar]
- 48. Nguyen T. T., Elsner R. A., and Baumgarth N. (2015) Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J. Immunol. 194, 1489–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dunn R., Hayashi S., Gillam I. C., Delaney A. D., Tener G. M., Grigliatti T. A., Kaufman T. C., and Suzuki D. T. (1979) Genes coding for valine transfer ribonucleic acid-3b in Drosophila melanogaster. J. Mol. Biol. 128, 277–287 [DOI] [PubMed] [Google Scholar]
- 50. Haas M. S., Alicot E. M., Schuerpf F., Chiu I., Li J., Moore F. D., and Carroll M. C. (2010) Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc. Res. 87, 618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edgerton C., Crispín J. C., Moratz C. M., Bettelli E., Oukka M., Simovic M., Zacharia A., Egan R., Chen J., Dalle Lucca J. J., Juang Y. T., and Tsokos G. C. (2009) IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin. Immunol. 130, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shigematsu T., Wolf R. E., and Granger D. N. (2002) T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia-reperfusion. Microcirculation 9, 99–109 [DOI] [PubMed] [Google Scholar]
- 53. Watson M. J., Ke B., Shen X. D., Gao F., Busuttil R. W., Kupiec-Weglinski J. W., and Farmer D. G. (2012) Treatment with antithymocyte globulin ameliorates intestinal ischemia and reperfusion injury in mice. Surgery 152, 843–850 [DOI] [PubMed] [Google Scholar]
- 54. Gimferrer I., Farnós M., Calvo M., Mittelbrunn M., Enrich C., Sánchez-Madrid F., Vives J., and Lozano F. (2003) The accessory molecules CD5 and CD6 associate on the membrane of lymphoid T cells. J. Biol. Chem. 278, 8564–8571 [DOI] [PubMed] [Google Scholar]
- 55. Jordö E. D., Wermeling F., Chen Y., and Karlsson M. C. (2011) Scavenger receptors as regulators of natural antibody responses and B cell activation in autoimmunity. Mol. Immunol. 48, 1307–1318 [DOI] [PubMed] [Google Scholar]
- 56. Wortis H. H., Teutsch M., Higer M., Zheng J., and Parker D. C. (1995) B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc. Natl. Acad. Sci. U.S.A. 92, 3348–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kretschmer K., Jungebloud A., Stopkowicz J., Stoermann B., Hoffmann R., and Weiss S. (2003) Antibody repertoire and gene expression profile: implications for different developmental and functional traits of splenic and peritoneal B-1 lymphocytes. J. Immunol. 171, 1192–1201 [DOI] [PubMed] [Google Scholar]
- 58. Chumley M. J., Dal Porto J. M., and Cambier J. C. (2002) The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J. Immunol. 169, 1735–1743 [DOI] [PubMed] [Google Scholar]
- 59. Dal Porto J. M., Burke K., and Cambier J. C. (2004) Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity 21, 443–453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.