Figure 3. Binding of ADP induces no further conformational changes.
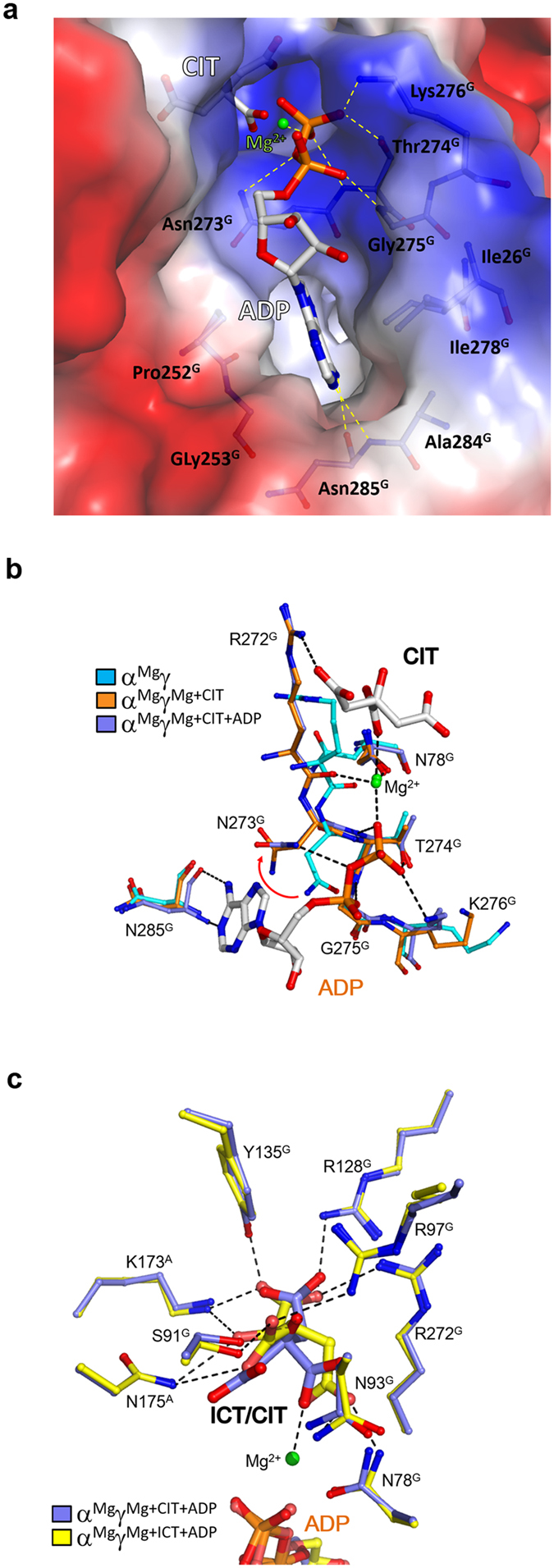
(a) Structure of the ADP-binding subsite in the αMgγMg+CIT+ADP structure. The protein is shown with electrostatic potential surface, the bound CIT and ADP are shown with ball-and-stick models, the Mg2+ with a green sphere, and the surrounding residues with side chains. The hydrophilic interactions of ADP with the surrounding residues and the Mg2+ are indicated with dashed lines. (b) Comparison of the ADP-binding subsite in the αMgγ (cyan), αMgγMg+CIT (orange) and αMgγMg+CIT+ADP (slate) structures. In the αMgγMg+CIT and αMgγMg+CIT+ADP structures, the side chain of Asn273G rotates about 100° away from the ADP-binding subsite compared to that in the αMgγ structure. (c) Comparison of the CIT-binding subsite in the αMgγMg+ICT+ADP (yellow) and αMgγMg+CIT+ADP (slate) structures. The bound ICT, CIT and ADP are shown with ball-and-stick models, the Mg2+ with a green sphere, and the surrounding residues with side chains. The hydrophilic interactions of ICT with the surrounding residues and the Mg2+ are indicated with dashed lines. ICT binds to the CIT-binding subsite and induces similar conformational changes.
