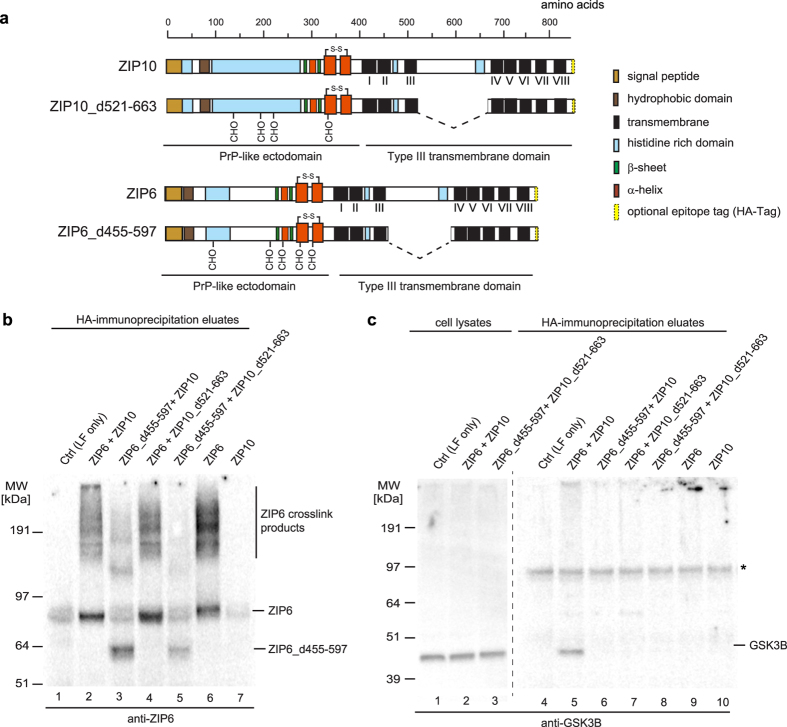
Figure 9. GSK3 binds to scaffold formed by histidine-rich cytoplasmic loop domains within the ZIP6-ZIP10 heteromer.
(a) Domain organization of ZIP10 and ZIP6 and deletion constructs used in this work to test the hypothesis that a cytoplasmic loop connecting transmembrane (TM) domains III and IV is essential for binding to GSK3 binding. (b) Control Western blot documenting ZIP6 expression products in eluates shown in panel ‘e’. (c) The co-expression of wild-type ZIP6 and ZIP10 proteins comprising intact cytoplasmic loop domains connecting TM domains III and IV is essential for binding of GSK3. GSK3-directed Western blot analysis of cellular lysates and co-immunoprecipitation eluates from in vivo formaldehyde crosslinked cells expressing different combinations of wild-type and/or mutant ZIP6/ZIP10. Note that in addition to protein bands reflecting the expected monomeric molecular weights of GSK3 and ZIP6, the Western blots in panels ‘d’ and ‘e’ revealed the appearance of high molecular weight crosslinked bands, whose appearance depended on the application of the in vivo formaldehyde crosslinking step.