Fig. 2.
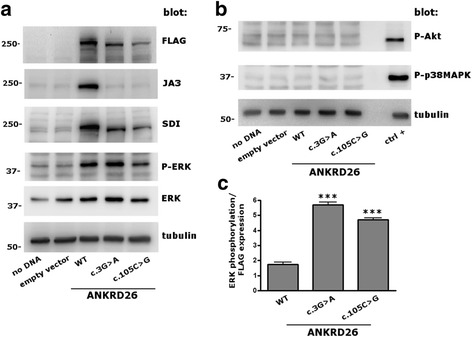
The c.3G>A and c.105C>G variant result in the synthesis of N-terminal truncated proteins that maintain the ability to phosphorylate ERK. ANKRD26-FLAG wild-type (WT) or mutant (c.3G>A or c.105C>G) constructs, or the empty vector, were transfected into HeLa cells. A further control was performed by avoiding DNA loading during transfection (no DNA). a Cells were lysed 48 h after transfection and an aliquot of 20 μg of protein was analyzed by immunoblotting. Transfection of both mutant constructs resulted in bands running at a slightly lower molecular mass compared to the WT band, which were recognized by the anti-FLAG and the SDI antibodies, but not by the JA3 antibody. Moreover, transfection of the WT as well as the mutant proteins (but not of the empty vector) induced the phosphorylation of ERK. Tubulin was used as loading control. b Transfection of WT or mutant ANKRD26 had no effects on phosphorylation of signaling kinases AKT or p38-MAPK. A lysate of platelets stimulated by 10 μM TRAP was used as positive control (ctrl+). c The ability of transfected ANKRD26-FLAG in phosphorylating ERK was measured as the P-ERK/ERK ratio weighted for the amount of FLAG, as determined by densitometric analysis of the respective bands. This value was significantly higher for both mutants compared to WT ANKRD26 (***P < 0.001). Data reported represent the mean of three independent experiments and are reported as mean ± S.E.M. Statistical analysis was performed by Student t test
