Abstract
Aim
The adequacy of 50 mcg folic acid supplementation given to low-birth-weight babies was investigated. The folate levels of the mothers and infants, and breastmilk, and the optimum dose for folic acid supplementation were also investigated.
Material and Methods
After obtaining blood from 141 low-birth-weight infants on the 1st day of life for serum and red cell folate levels, the infants were randomly allocated into three groups according to the folic acid supplement dose. Forty-six infants were given 25 μg/d folic acid, 39 were given 50 μg/d folic acid, and 44 were given 75 μg/d folic acid. Folic acid could not be given to 12 infants. Follow-up blood samples were obtained at the end of folic acid supplementation. Maternal samples for red cell and serum folate levels and breast milk folate levels were obtained within the first 48 hours and the samples for measuring breastmilk folate level were obtained on the 3rd day postnatally. The feeding modes of the infants, maternal folic acid intake, and details of neonate intensive care unit course were recorded.
Results
The mean birth weight and gestational age of the infants were found as 1788.2±478.4 g and 33.5±2.9 weeks, respectively. The mean serum and red cell folate levels on admission were found as 21.2±12.2 ng/mL and 922.7±460.7 ng/mL, respectively. The mean maternal serum and red cell folate levels and the mean breast milk folate levels were found as 12.3±7.5 ng/mL, 845.5±301.4 ng/mL, and 30.6±33.0 ng/m, respectively. The breast milk folate levels of mothers who were supplemented with folic acid during pregnancy were significantly higher compared with mothers who were not supplemented with folic acid (p<0.001). Infants who were supplemented with folic acid had higher follow-up serum folate levels compared with the basal level in all groups, but there was no statistically significant difference between the groups.
Conclusion
This study showed that the folic acid doses of 25, 50, and 75 μcg/d affected serum folate levels similarly. We can conclude that the dose of 25 μcg/d is adequate for low-birth-weight infants.
Keywords: Breast milk, low-birth-weight infant, folic acid, supplementation, newborn
Introduction
Folate is a water-soluble vitamin that cannot be synthesized in the human body. Co-enzymes that contain folate are involved in cell renewal/differentiation, DNA synthesis, methionine synthesis, and hematopoesis. The folate balance in the body depends on intake, destruction, and excretion in urine and stool. If not received regularly, folate deficiency occurs in 2–3 months. Folate deficiency leads to megaloblastic anemia (1).
The erythrocyte folate level (RBCFL) is higher in preterm and term babies compared with adults because fetal folate transfer is increased in pregnancy (especially in the third trimester) (2–5). Folate decreases gradually in the first months of life, predominantly in low-birth-weight (LBW) babies. In this rapid growth period of life, requirement of folic acid (FA) increases and the risk of development of folate deficiency is increased. Studies conducted in the 1970s–80s reported that folate levels in preterm babies decreased in the first 2–3 months of life (3, 4). Therefore, routine FA supplementation was recommended for all preterm babies in the 1990s (6). In addition to preterm babies, FA supplementation of 20–50 μcg/day was recommended for babies with a birth weight of <2 500 g (7, 8). In our unit, FA supplementation of 20–50 μcg/day is given to LBW babies. However, the folate levels of the babies were not considered when proposing this recommendation. The mode of feeding for the baby [breastmilk (BM), formula, fortified breastmilk (FBM) or total parenteral nutrition] and/or the folate level of the baby at the time of birth affects the requirement for folate. Unfortunately, there are a limited number of studies related with normal blood folate levels in the neonatal period comprising heterogeneous groups (9).
Owing to the inadequacy of the current information related with FA supplementation dose and its necessity in low-birth-weight babies, the following questions were investigated:
Is the recommended FA dosage of 50 mcg/day appropriate for all babies?
Does the mode of nutriton affect requirement for FA?
What is the most appropriate FA dose?
What are the maternal serum, eryhtrocyte, BM levels of FA in the 2000s?
Material and Methods
After written informed consent was obtained from the families, LBW babies who were born in Ondokuz Mayıs University (OMU) Hospital between 01.01.2012 and 09.31.2012 and followed up in the neonatal intensive care unit (NICU) were included in this prospective study. Blood samples were obtained from the babies after admission in the ward for serum/RBCFL and hematocrit levels. Afterwards, the babies were divided into three groups using a sealed tender and accordingly given 25 μcg, 50 μcg, and 75 μcg FA supplementation daily. The prenatal, natal, and postnatal characteristics of the babies were recorded. The modes of daily feeding (BM and/or formula, SBM) and the amount of FA supplementation were recorded. Folic acid supplementation was discontinued when the baby became 2 500 g or one month of age (whichever occured first). At the end of supplementation, blood samples were obtained from the babies for follow-up serum/RBCFL and hematocrit values. Blood samples from the mothers for serum/RBCFL and hematocrit values were obtained in the first 48 hours in the postnatal period. The samples for BM folate levels were obtained in the first 72 hours. Prenatal maternal FA intake was recorded.
According to the protocol applied in our unit, BM fortifier was initiated when the baby was receiving 50 mL/kg BM. Breastmilk fortifier was prepared by adding 3 spoons of BM fortifier (Eoprotin) into 100 mL BM. The fortified BM contains 50 μg/100 mL FA and the preterm formula used in our unit (Prematil) contains 28 μg/100 mL FA.
Ethics committee approval was obtained for the study (Number: 2011/339) and financial support was obtained from Ondokuz Mayıs University (PYO.TIP.1904.11.032). Financial support was obtained from the drug company Berko for studying BM folate levels.
Biochemical Tests
In our study, RBCFL was also studied in addition to folate level because studies have reported that RBCFL gives better information about body stores (10). The samples were placed in regular tubes and K3-EDTA tubes. The samples in the regular tubes were centrifuged for 5 minutes at 3000 g and kept in polystyrene tubes at −50°C. No procedure was applied for the samples placed in K3-EDTA tubes and they were kept at −50°C.
For measurement of the total blood folate level, 0.2% ascorbic acid solution was prepared to disintegrate the erythrocytes inside the K3-EDTA tubes. For preparation of 0.2% ascorbic acid, one bag among the 0.4 g ascorbic acid bags in the ROCHE RBC Folate Hemolyzing kit was poured into the 200 mL plastic container found in the same kit. Deionized water (200 mL) was added to the container and mixed after waiting for 5 minutes at room temperature.
Three milliliters of 0.2% ascorbic acid solution was placed in 5 mL polystyrene tubes. After it was dissolved at room temperature and mixed by reversing, 100 microliters were removed from the blood in the K3-EDTA tube and added to the prepared ascorbic acid solution. After these tubes were reversed three times, they were incubated at room temperature for 90±15 minutes. The RBCFL was measured for disintegration of the erythrocytes. The complete blood folate level was studied using competitive electrochemiluminescence immunoassay (ECLIA) with the Modular Analytics E170 device. The following formula was used for measurement of ERBC folate level:
A value of >140 ng/mL was considered normal for RBCFL (10).
For analysis of serum folate level, the frozen samples were dissolved at room temperature and measurements were performed using competitive ECLIA with a Modular Analytics E170 device. As in previous studies, a value of <3 ng/mL was considered the limit value for folate deficiency (11, 12).
The lipid in BM was eliminated to measure the BM folate concentration. The “Extraction of the Lipemic Serum Samples with Chloroform” protocol used in lipemic serum samples was applied. With this objective, 600 μL chloroform was mixed with 300 μL BM and vortexed for one minute. Afterwards, it was centrifuged for 5 minutes at 4°C and 16 000 g. The supernatant was removed and 1/dilution was performed with diluent universal. Folate measurement was performed using copetitive chemiluminescence with a Modular Analytics E 170 device.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 15.0 (SPSS Inc.; Chicago, IL, USA). The frequencies in the groups were selected as 46, 46, and 46 with a 95% strength at a confidence interval of 95% such that d=5 difference was found with 6 deviations. Data obtained through measurements are expressed as mean±SD and data obtained by counting are expressed as percentage. The variables did not all show normal distribution. Therefore, nonparametric tests were used in all hypothesis tests. The Wilcoxon signed-rank test was used in the comparison of basal values with the control values. Based on these differences, the Kruskal-Wallis test was used to determine whether the changes in the groups were significant. Non-parametric Spearman correlation coefficients were used for correlation measurements. Correlations were calculated for all groups by excluding the groups one by one and for each group separately. The Mann-Whitney U test was used in comparison by intake of folic acid in pregnancy. In the comparison of categorical variables between the groups, Fischer’s exact test and the Chi-square test were used. A p value of <0.05 was considered significant.
Results
One hundred ninety-four LBW babies were followed up in the NICU during the study period. Thirteen babies were excluded from the study and 19 babies were lost. Four babies were excluded because of Down syndrome, eight babies were excluded because of major congenital anomalies, and one baby was excluded because of epidermolysis bullosa. One hundred sixty-two babies were eligible for the study; 18 babies could not be included in the study because their families did not give consent. One baby was not present for a follow-up visit and thus a blood sample could not be obtained from this baby. The blood tubes of two babies were broken. In total, 141 LBW babies were included in the study. The prenatal, natal, and postnatal characteristics of the babies were recorded. After randomization, 46 babies received 25 μcg FA supplementation (group 1), 39 babies received 50 μcg FA supplementation (group 2), and 44 babies received 75 μcg/g FA supplementation (group 3). A control group was not planned because a group that would not receive FA would not be ethical. However, 12 babies could not receive folic acid because of enteral feeding problem. There were nine twins and one set of triplets (130 mothers). A nutrition questionnaire related with nutrition during pregnancy was given to the mothers and all mothers reported that they ate folate-rich food during pregnancy. None of the mothers used alcohol or smoked. The characteristics of the babies, folate levels at birth, maternal characteristics, maternal serum FA levels, RBCFLs, and BM folate levels are shown in Table 1 and 2. The modes of nutrition of the babies were as follows: BM alone 10.6%, BM plus formula 60.9%, FBM 8.2%, formula alone 5.8%, and FBM alone 14.5%. There was no diffference in terms of nutrition mode between the three groups (p=0.058).
Table 1.
The characteristics and folate levels of the babies and mothers
| Group 1 FA (−) (n=12) | Group 2 (25 μg/g) (n=46) | Group 3 (50 μg/g) (n=39) | Group 4 (75 μg/g) (n=44) | General (n=141) | |
|---|---|---|---|---|---|
|
| |||||
| (mean±SD) | (mean±SD) | (mean±SD) | (mean±SD) | (mean±SD) | |
| Min–max | Min–max | Min–max | Min–max | Min–max | |
| (median) | (median) | (median) | (median) | (median) | |
| Gestational age (weeks) | 3.19±4.5 | 33.4±2.6 | 33.8±2.5 | 33.8±3.1 | 33.5±2.9 |
| (25–40) | (27–39) | (28–39) | (26–41) | (25–41) | |
| (32) | (34) | (34) | (34) | (34) | |
| p | 0.396 | ||||
| Birth weight (g) | 1 533.3±701.8 | 1 795.2±445.5 | 1 843.1±406.6 | 1 801.8±494.2 | 1 788.2±478.4 |
| (700–2 390) | (840–2 410) | (950–2 420) | (790–2 430) | (700–2 430) | |
| (1 315) | (1 920) | (1 880) | (1 870) | (1 880) | |
| p | 0.640 | ||||
| Maternal age (years) | 28.2±6.2 | 27.4±5.9 | 27.8±6 | 28.2±6.6 | 27.9±6.1 |
| (21–39) | (17–41) | (14–39) | (18–42) | (14–42) | |
| (27) | (26.5) | (27.5) | (27) | (27) | |
| p | 0.913 | ||||
| Maternal RBCFL level (ng/mL) | 913.0±290.7 | 810.4±288.1 | 848.6±288.7 | 858.3±331 | 845.5±3 01.4 |
| (572–1 431) | (359–1 362) | (315–1 520) | (294–2 003) | (294–2 003) | |
| (870) | (775) | (868.5) | (780) | (798) | |
| p | 0.851 | ||||
| Maternal serum folate level (ng/mL) | 13.7±8.1 | 12.1±7.5 | 13.5±7.1 | 11.9±7.8 | 12.3±7.5 |
| (2.8–28.5) | (1.9–32.2) | (1.5–39.9) | (2.4–33.2) | (1.5–39.9) | |
| (13.6) | (12.1) | (13.4) | (8.8) | (11.3) | |
| p | 0.390 | ||||
| Breastmilk folate level (ng/mL) | 49.4±50.5 | 28.3±33.8 | 32.2±30.8 | 28.9±31.6 | 30.6±33.0 |
| (4.9–165.6) | (5.6–144) | (5.8–141) | (6–165) | (4.6–165.6) | |
| (26.7) | (13.7) | (21.1) | (17.5) | (17.0) | |
| p | 0.116 | ||||
| Neonatal basal RBCFL level (ng/mL) | 835.2±144 | 916.5±392.7 | 956.9±582.5 | 922.7±470.3 | 922.7±460.7 |
| (692–1 145.9) | (285.5–2 058.5) | (398.1–3 864.3) | (419.6–2 994.6) | (285.6–3 864.4) | |
| (781.5) | (839.2) | (825.9) | (850.2) | (841.8) | |
| p | 0.886 | ||||
| Neonatal basal serum folate level (ng/mL) | 20.1±9.4 | 21.5±15.7 | 21.6±10.5 | 20.7±10.1 | 21.2±12.2 |
| (6.8–35.2) | (4.1–90) | (6.7–53.9) | (4.9–48.1) | (4.1–90) | |
| (18.4) | (17.3) | (18.8) | (19.1) | (18.5) | |
| p | 0.965 | ||||
Mean±SD: mean±standard deviation; RBCFL: red blood cell folate level
Table 2.
Demographic properties of the babies
| Group 1 FA (−) (n= 12) | Group 2 (25 μg/g) (n=46) | Group 3 (50 μg/g) (n=39) | Group 4 (75 μg/g) (n=44) | General | |
|---|---|---|---|---|---|
| Gender (M/F) n (% ) | 8 (66.7)/4 (33.3) | 22 (47.8)/24 (52) | 16 (41)/23 (59) | 20 (45.5)/24 (54.5) | 66 (46.8)/75 (53.2) |
| p | 0.480 | ||||
| SGA n (%) | 6 (50) | 18 (39.1) | 18 (46.2) | 22 (50) | 64 (45.3) |
| p | 0.749 | ||||
| Exclusively breasfed n (%) | 0 (0) | 6 (13) | 7 (18.4) | 2 (4.8) | 15 (10.6) |
| p | 0.132 | ||||
| FA intake during pregnancy n (%) | 11 (91.7) | 40 (87) | 34 (87.2) | 40 (90.9) | 125 (88.6) |
| p | 0.909 | ||||
| Pre-eclampsia n (%) | 4 (33.3) | 18 (39.1) | 11 (28.2) | 13 (29.5) | 46 (32.6) |
| p | 0.814 | ||||
| TPN n (%) | 9 (75) | 27 (58.7) | 21(53.8) | 31 (70.5) | 88 (62.4) |
| p | 0.319 | ||||
| Hospitalization for blood transfusion n (%) | 0 | 4 (8.7) | 0 | 2 (4.5) | 6 (4.3) |
| p | 0.638 |
FA: folic acid; F: female; M: male; RBCFL: red blood cell folate level; SGA: small-for-gestational age; TPA: total parenteral nutrition
The serum folate levels were <3 ng/mL in four mothers and >3 ng/mL in all babies. The RBCFLs were found normal in all mothers including those with low serum folate levels. None of the mothers were diagnosed as having folate deficiency.
A weak statistically significant positive correlation was found between maternal RBCFL and maternal serum folate level (p<0.001, r2=14.5%).
A weak statistically significant positive correlation was found between maternal RBCFL and basal neonatal RBCFL (p<0.001, r2=15.4).
A weak statistically significant positive correlation was found between maternal RBCFL and the basal neonatal serum folate level (p=0.012, r2=3.6%).
No statistically significant correlation was found between the maternal RBCFL and BM folate levels (p=0.366).
No statistically significant correlation was found between the maternal serum folate level and basal neonatal RBCFL (p=0.206).
A weak statistically significant positive correlation was found between the maternal folate level and the basal neonatal serum folate level (p<0.001, r2=17.3%). A weak statistically significant positive correlation was found between the maternal serum folate level and BM folate level (p=0.009, r2=3.9%).
There was no significant correlation between the maternal RBCFL, maternal serum folate level, and the birth weights of the babies (p=0.466 for RBCFL, p=0.625 for serum) and prenatal delivery (p=0.967 for RBCFL, p=0.651 for serum).
No significant correlation was found between the use of FA in pregnancy and the basal neonatal RBCFL and basal neonatal serum level (p=0.511 for RBCFL, p=0.398 for serum).
The BM folate levels were found statistically significantly higher in mothers who used FA during pregnancy compared with those who used no FA during pregnancy (p<0.001, Figure 1).
Figure 1.
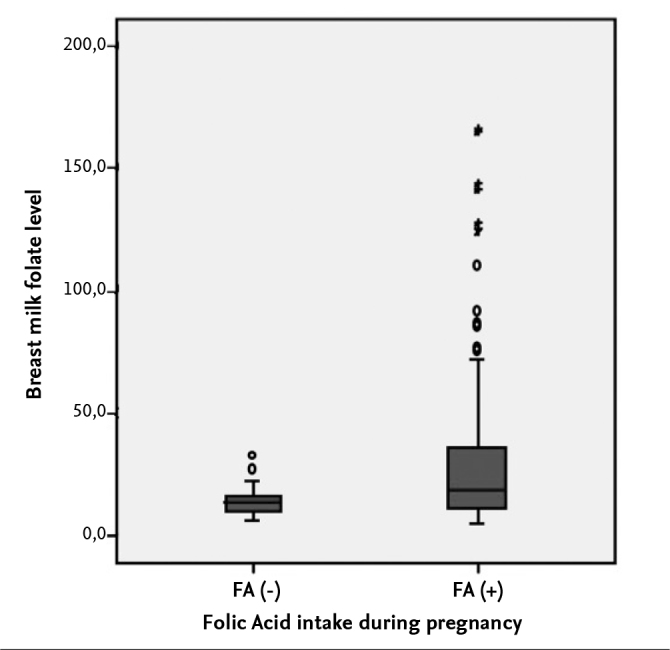
Maternal use of folic acid during pregnancy and breastmilk folate level
Although the RBCFL and serum folate levels of the preeclamptic mothers were found lower compared with those who were not preeclamptic, the difference was not statistically significant (p=0.758 for RBCFL, p=0.638 for serum).
No significant correlation was found between the basal neonatal RBCFL and basal neonatal serum folate levels and gestational week, birth weight, respiratory distress syndrome, bronchopulmonary displasia, retinopathy of prematurity, intraventricular hemorrhage, culture positive sepsis, congenital heart disease or rehospitalization for erythrocyte transfusion (p>0.05 for all variables).
There was no difference between the groups that received different doses of FA supplementation in terms of demographic properties (Tables 1 and 2). There was also no difference between the three groups in terms of maternal RBCFL, serum, basal neonatal RBCFL, serum or BM folate level (Table 1).
No statistically significant difference was found between babies who received different doses of FA supplementation in terms of basal serum folate levels (p=0.965).
In all three groups that were given folic acid, the follow-up serum folate levels were found higher compared with basal levels, but there was no difference between the groups (p=0.354) (Table 3).
Table 3.
Intra- and between-groups comparison of the follow-up folate levels of the groups given folic acid
| Group 1 (25 μg/g) (n=46) | Group 2 (50 μg/g) (n=39) | Group 3 (75 μg/g) (n=44) | |
|---|---|---|---|
|
| |||
| (mean±SD) | (mean±SD) | (mean±SD) | |
| Min–max, | Min–max, | Min–max, | |
| (median) | (median) | (median) | |
| Neonatal basal RBCFL level (ng/mL) | 916.5±3927 | 956.9±582.5 | 922.7±470.3 |
| (285.5–2058.5) | (398.1–3864.3) | (419.6–2994.6) | |
| (839.2) | (825.9) | (850.2) | |
| Neonatal follow-up RBCFL level (ng/mL) | 1152.3±426.7 | 1176.7±350.3 | 1187.5±744.9 |
| (567–3145) | (337.1–2076) | (534.4–5483.8) | |
| (1109.2) | (1132.5) | (1054.5) | |
| P (p values belonging to the differences) | 0.003 | 0.002 | <0.001 |
| p (p values for comparison of the differences by groups) | 0.599 | ||
| Neonatal basal serum folate level (ng/mL) | 21.5±15.7 | 21.6±10.5 | 20.7±10.1 |
| (4.1–90) | (6.7–53.9) | (4.9–48.1) | |
| (17.3) | (18.8) | (19.1) | |
| Neonatal follow-up serum folate level (ng/mL) | 35.3±18.6 | 35.1±16.8 | 35.4±16.1 |
| (6.8–78.2) | (8.4–75.6) | (6.2–82.6) | |
| p (p values of the differences) | <0.001 | <0.001 | <0.001 |
| p (p values for comparison of the differences by groups) | 0.354 | ||
Mean ±SD: mean±standard deviation; RBCFL: red blood cell folate level
No difference was found between the groups in terms of rehospitalization for erythrocyte transfusion (p=0.638).
No significant correlation was found between the mode of feeding and follow-up serum folate levels (p=0.753). 213 Turk Pediatri Ars 2016; 51: 210–6 Çakmak Çelik et al. Folic acid supplementation for low-birth-weight babies
Discussion
In this study, the requirement for FA supplementation was investigated in LBW babies. In one study, 29 LBW babies were examined and the mean RBCFL level was found as 563 ng/mL (range, 230–1250 ng/mL) and the mean plasma folate level of 18 babies was reported as 18.7 ng/mL (range, 5.5–30 ng/mL) (13). In our study, the mean RBCFL and serum folate levels on the first day of life were 922.7±460.7 ng/mL and 21.2±12.2 ng/mL, respectively. The basal RBCFL and serum folate levels were not low in any babies. In a study conducted in Turkey, the mean serum folate level was reported as 11.5–71.7 ng/mL in 162 babies with a gestational age of ≤ 32 weeks (14). The serum folate and RBCFL levels in the study of Roberts et al. (13) and the serum folate levels in the study of Öncel et al. (14) were similar to our study. Accordingly, we can state that LBW babies have normal folate levels at the time of birth.
In a study conducted with 35 babies who were fed with BM, preterm babies who received 65 μg/g FA supplementation were compared with preterm babies who did not receive FA supplementation and the RBCFL levels were reported lower in those who did not receive FA supplementation. The mean RBCFL level was reported as 257.4±31.3 ng/mL in the group that received FA supplementation. In the same study, it was reported that RBCFL and plasma folate levels were reported higher in babies compared with adults; none of the mothers used FA during pregnancy (3). In our study, the follow-up serum folate levels of babies who received FA supplementation were found high in each group, but there was no difference between the groups. When more exogeneous FA is received than is required by the body, urinary excretion is increased and excess FA is eliminated from the body. The results of our study suggest that this mechanism is also developed in LBW newborns.
In our study, the follow-up serum and RBCFL levels in 12 babies who did not receive FA supplementation were found as 32.8±34.5 ng/mL and 1054.4±395 ng/mL, respectively. As in the other groups that did not receive folic acid supplementation, FA deficiency did not develop at the end of the first month in the group that did not receive FA.
In a study conducted in 1966, it was reported that the RBCFL levels of babies were higher than their mothers (15). In our study, the RBCFL and serum folate levels of the babies were found higher compared with their mothers, in aggreement with the study conducted in 1966.
One of the questions that we tried to answer in our study was as follows: What are the maternal RBCFL, serum, and BM folate levels in the 2010s? The maternal serum and RBCFL levels were found as 12.3±7.5 ng/mL and 845.5±301.4 ng/mL, respectively. Folate deficiency was not found in any mother.
Ramasastri (16) reported the maternal BM folate level as 4.4 ng/mL for colostrum (14 milk samples) and 8.4 ng/mL for transition milk (5–15 days) in nine maternal BM samples in 1965. In that study, which did not state whether the babies were term or preterm, vitamin supplementation was not given to any of the mothers. Cooperman (17) reported that the mean colostrum folate level was 15.2 ng/mL (range, 4–33.2 ng/mL) in 70 BM samples obtained from 15 mothers in 1982. In another study, in which folate was studied in maternal BM samples obtained from 20 mothers who received 3.4 mg/day FA starting from the beginning of pregnancy, the BM folate level was reported as 0.3–3.9 ng/mL (13). In our study, the mean BM folate level (considered colostrum, because it was obtained in the first three days) was found as 30.6±33.0 ng/mL (range, 4.6–165.6 ng/mL) (medan 17 ng/mL). The fact that the maternal BM folate was found higher compared with the other studies may be related with alterations in nutritional behaviors of mothers in the intervening forty-year period. In addition, a weak correlation was observed between the use of FA in pregnancy and maternal folate levels in our study. Therefore, maternal BM folate might have been found higher compared with the other studies. In the study conducted by Tamura et al. (18), BM folate in 16 mothers was reported as 62–280 ng/mL and it was found that the serum folate level of the baby and folate levels in maternal BM were correlated. However; the authors did not specify which day the BM was obtained. In our study, no correlation was found between maternal BM folate and the follow-up serum folate of the baby.
In a study of 91 mothers, no significant correlation was found between maternal BM folate and the mother’s RBCFL and plasma folate at any stage of lactation (19). Similarly, we found no correlation between maternal RBCFL and BM folate, but there was a weak statistically significant positive correlation between maternal serum folate and BM folate.
In two studies in which babies fed with preterm formula or FBM were evaluated in terms of FA supplementation, it was reported that these babies did not require FA supplementation (14, 20).
In our study, no significant difference was found between the groups in terms of the follow-up serum folate level according to feeding mode. The FA supplementation requirement of the babies who were fed with BM alone could not be evaluated in our study because we had no control group with BM feeding alone without FA supplementation.
With this study, it can be stated that the dosage of 25 mc/g is adequate for FA supplementation. In conclusion, the mode of feeding of the baby should be considered when specifying the need for FA supplementation in babies with a weight <2500 g. FA supplementation may not be needed in LBW babies fed with fortified BM or preterm formula and in babies of mothers who have a balanced diet and received folic acid supplementation during pregnancy. Further studies are needed to determine the necessity of FA supplementation in LBW babies who are exclusively fed with BM.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from local ethic committee (No: 2011/339).
Informed Consent: Written informed consent was obtained from parents of patients who participated in this study.
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: Financial grant was obtained from Ondokuz Mayıs University (PYO.TIP.1904.11.032). Berko Pharmaceutical financed (500 Euro) the analysis of breast milk folate levels.
Author Contributions: Concept - F.C.C., C.A.; Design - F.C.C., C.A., A.B.; Supervision - C.A., S.K.; Funding - F.C.C, C.A., S.K.; Materials - F.C.C., S.G.; Data Collection and/or Processing - F.C.C., S.G.; Analysis and/or Interpretation - F.C.C., C.A., S.G., A.B., E.C., Y.B.; Literature Review - F.C.C., C.A.; Writing - F.C.C.; Critical Review - C.A., S.K.
References
- 1.Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4:236–41. doi: 10.3121/cmr.4.3.236. https://doi.org/10.3121/cmr.4.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ek J. Plasma and red cell folate values in newborn infants and their mothers in relation to gestational age. J Pediatr. 1977;96:288–92. doi: 10.1016/s0022-3476(80)80497-5. [DOI] [PubMed] [Google Scholar]
- 3.Ek J, Magnus E. Plasma and red cell folate in breastfed infants. Acta Paediatr Scand. 1979;68:239–43. doi: 10.1111/j.1651-2227.1979.tb04995.x. https://doi.org/10.1111/j.1651-2227.1979.tb04995.x. [DOI] [PubMed] [Google Scholar]
- 4.Ek J, Behnecke L, Halvorsen KS, Magnus E. Plasma and red cell folate values and folate requirements in formula-fed premature infants. Eur J Pediatr. 1984;142:78–82. doi: 10.1007/BF00445582. https://doi.org/10.1007/BF00445582. [DOI] [PubMed] [Google Scholar]
- 5.Vanier TM, Tyas JF. Folic acid status in pre-mature infants. Arch Dis Child. 1967;42:57–61. doi: 10.1136/adc.42.221.57. https://doi.org/10.1136/adc.42.221.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuller NJ, Bates CJ, Cole TJ, Lucas A. Plasma folate levels in preterm infants, with and without a 1 mg daily folate supplement. Eur J Pediatr. 1992;151:48–50. doi: 10.1007/BF02073891. https://doi.org/10.1007/BF02073891. [DOI] [PubMed] [Google Scholar]
- 7.Matoth Y, Zamir R, Bar-Shani S, Grossowicz N. Studies on folic acid in infancy. II. Folic and folinic acid blood levels in infants with diarrhea, malnutrition, and infection. Pediatrics. 1964;33:694–9. [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976–86. [PubMed] [Google Scholar]
- 9.Burland WL, Simpson K, Lord J. Response of low birthweight infant to treatment with folic acid. Arch Dis Child. 1971;46:189–94. doi: 10.1136/adc.46.246.189. https://doi.org/10.1136/adc.46.246.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu A, Chanarin I, Slavin G, Levi AJ. Folate deficiency in the alcoholic--its relationship to clinical and haematological abnormalities, liver disease and folate stores. Br J Haematol. 1975;29:469–78. doi: 10.1111/j.1365-2141.1975.tb01844.x. https://doi.org/10.1111/j.1365-2141.1975.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 12.Rice L. Laboratory diagnosis of vitamin B12 and folate deficiency. Arch Intern Med. 1999;159:2746–7. doi: 10.1001/archinte.159.22.2746-a. https://doi.org/10.1001/archinte.159.22.2746-a. [DOI] [PubMed] [Google Scholar]
- 13.Roberts PM, Arrowsmith DE, Rau SM, Monk-Jones ME. Folate state of premature infants. Arch Dis Child. 1969;44:637–42. doi: 10.1136/adc.44.237.637. https://doi.org/10.1136/adc.44.237.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oncel MY, Calisici E, Ozdemir R, et al. Is folic acid supplementation really necessary in preterm infants with ≤32 weeks of gestation? J Pediatr Gastroenterol Nutr. 2014;58:188–92. doi: 10.1097/MPG.0000000000000181. https://doi.org/10.1097/MPG.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 15.Grossowicz N, Izak G, Rachmilewitz M. The effect of anemia on the concentration of folate derivatives in paired fetal maternal blood. Isr J Med Sci. 1966;2:510–2. [PubMed] [Google Scholar]
- 16.Ramasastri BV. Folate activity in human milk. Br J Nutr. 1965;19:581–6. doi: 10.1079/bjn19650052. https://doi.org/10.1079/BJN19650052. [DOI] [PubMed] [Google Scholar]
- 17.Cooperman JM, Dweck HS, Newman LJ, Garbarino C, Lopez R. The folate in human milk. Am J Clin Nutr. 1982;36:576–80. doi: 10.1093/ajcn/36.4.576. [DOI] [PubMed] [Google Scholar]
- 18.Tamura T, Yoshimura Y, Arakawa T. Human milk folate and folate status in lactating mothers and their infants. Am J Clin Nutr. 1980;33:193–7. doi: 10.1093/ajcn/33.2.193. [DOI] [PubMed] [Google Scholar]
- 19.Ek J. Plasma, red cell and breast milk folacin concentrations in lactating women. Am J Clin Nutr. 1983;38:929–35. doi: 10.1093/ajcn/38.6.929. [DOI] [PubMed] [Google Scholar]
- 20.Jyothi S, Misra I, Morris G, Benton A, Griffin D, Allen S. Red cell folate and plasma homocysteine in preterm infants. Neonatology. 2007;92:264–8. doi: 10.1159/000103745. https://doi.org/10.1159/000103745. [DOI] [PubMed] [Google Scholar]


