Abstract
Plants are a source of complex bioactive compounds, with value as pharmaceuticals, or leads for synthetic modification. Many of these secondary metabolites have evolved as defenses against competing organisms. and their pharmaceutical value is “accidental”, resulting from homology between target proteins in these competitors, and human molecular therapeutic targets. Here we show that it is possible to use mutation and selection of plant cells to re-direct their “evolution” toward metabolites that interact with the therapeutic target proteins themselves. This is achieved by expressing the human target protein in plant cells, and selecting mutants for survival based on the interaction of their metabolome with this target. This report describes the successful evolution of hairy root cultures of a Lobelia species toward increased biosynthesis of metabolites that inhibit the human dopamine transporter protein. Many of the resulting selected mutants are overproducing the active metabolite found in the wild-type plant, but others overproduce active metabolites that are not readily detectable in non-mutants. This technology can access the whole genomic capability of a plant species to biosynthesize metabolites with a specific target. It has potential value as a novel platform for plant drug discovery and production, or as a means of optimizing the therapeutic value of medicinal plant extracts.
Keywords: Lobelia cardinalis; human dopamine transporter protein (hDAT); activation tagging mutagenesis (ATM); hairy root cultures; 1,2,3,6-tetrahydropyridine (MPTP)
1. Introduction
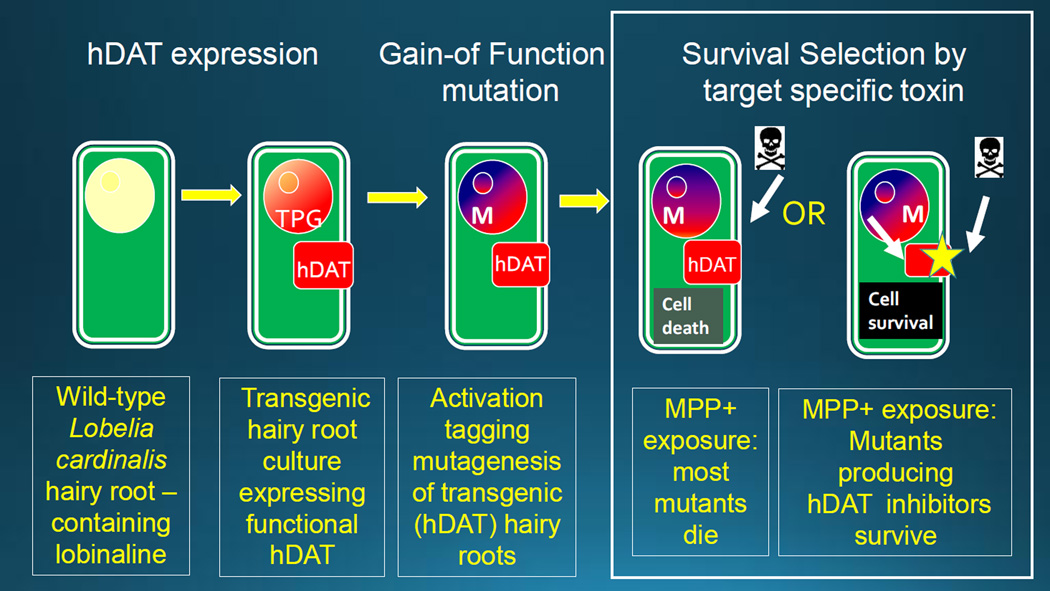
The pharmaceutical industry is increasingly abandoning plants as a source of drugs (see Littleton 2007). One reason is that many bioactive plant metabolites are too complex for convenient chemical synthesis, making them difficult to optimize for interaction with the molecular target. Another, is that production of such compounds requires purification from the variable and miniscule amounts in intact plants. A solution to the latter (pioneered by Phyton for taxanes) is to use cultured plant cells as production systems (Howat et al 2014). Here we show that mutant plant cells in culture can be used both as optimized production systems, and as sources of metabolites with optimized activity at the target. The concept is simple. First a plant species is identified that contains complex metabolites with activity at a molecular target protein. Then transgenic plant cells are created that stably express this target protein. Mutants of these transgenic cells are then selected under conditions that favor survival of individuals containing metabolites with the desired interaction with the target. The surviving sub-population is enriched in mutants containing metabolites with the appropriate pharmacological activity. These may be metabolites that are present in the wild-type plant or, if a mutation alters a relevant biosynthetic pathway, then “novel” active metabolites with enhanced activity at the target may be generated (Rogers et al 2003). Target proteins have previously been expressed in plant cells as screens (Littleton 2007, Doukhanina et al 2007, Zhao et al 2012, Gunjan et al 2013), but this is the first report in which survival of mutant plant cells expressing a foreign target protein has been used to direct secondary metabolism toward a specific pharmacological phenotype. Proof of concept uses Lobelia cardinalis, which contains lobinaline, a novel inhibitor of the human dopamine transporter (DAT) (Littleton et al 2004, Brown et al 2016). Metabolites with this activity have evolved in plants to inhibit similar transporter proteins in the brains of herbivorous insects to deter feeding (Chen et al 2006)). This homology makes them valuable as lead compounds in degeneration of dopaminergic neurons in the human brain (Rudnick et al 2014, Segura-Aguilar & Kostrzewa 2015). However, lobinaline, is a complex binitrogenous decahydroquinoline alkaloid (Manske 1938, Robison & Pierson 1964) with 5 chiral centers. Chemical synthesis is difficult, and lobinaline is present in low yields in the wild type plant. Lobinaline is therefore a poor candidate as a conventional lead for DAT inhibitors, but an excellent example for target-directed evolution of biosynthesis (see Figure 4).
Figure 4. The selection procedure for mutant transgenic hDAT hairy root cultures generated in 100µM MPP+.
Expression of the functional hDAT (transgenic protein-TPG) in hairy root cultures makes them susceptible to toxicity induced by MPP+, which is concentrated intracellularly by the hDAT. Therefore mutants (M) that overproduce inhibitors of the hDAT should have a survival advantage when exposed to MPP+. Although many mutants may survive by other mechanisms, the surviving population should therefore be “enriched” in individual mutants containing metabolites that inhibit the target protein.
2. Materials and methods
2.1 Expression of the functional target protein
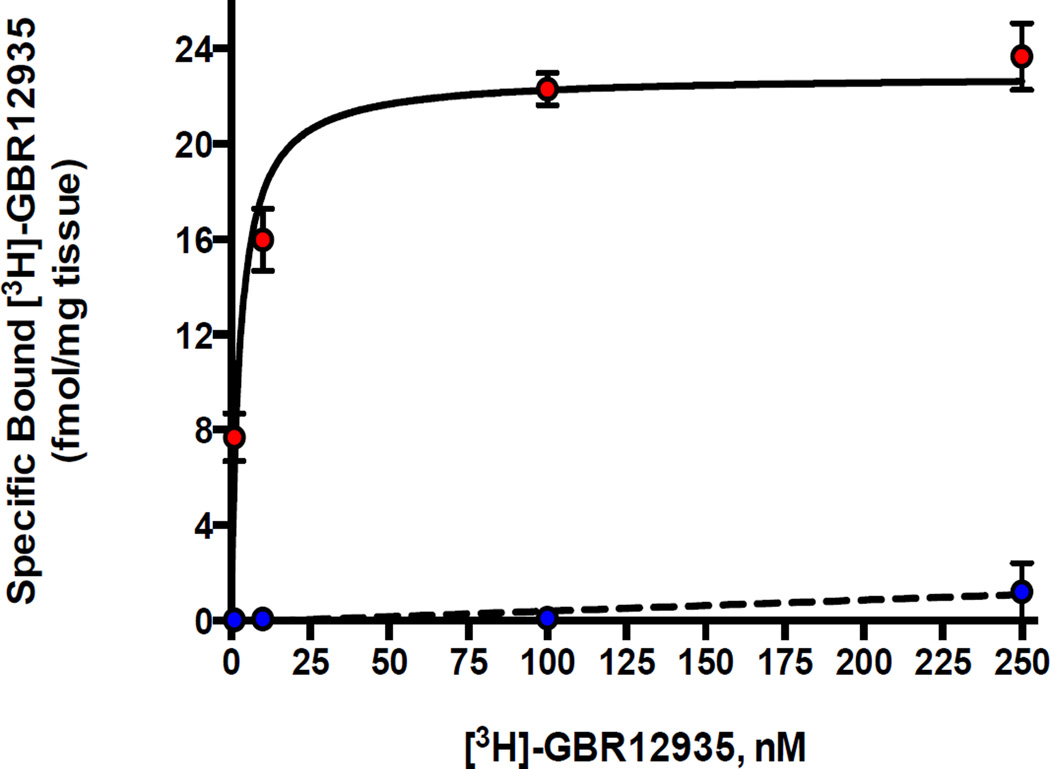
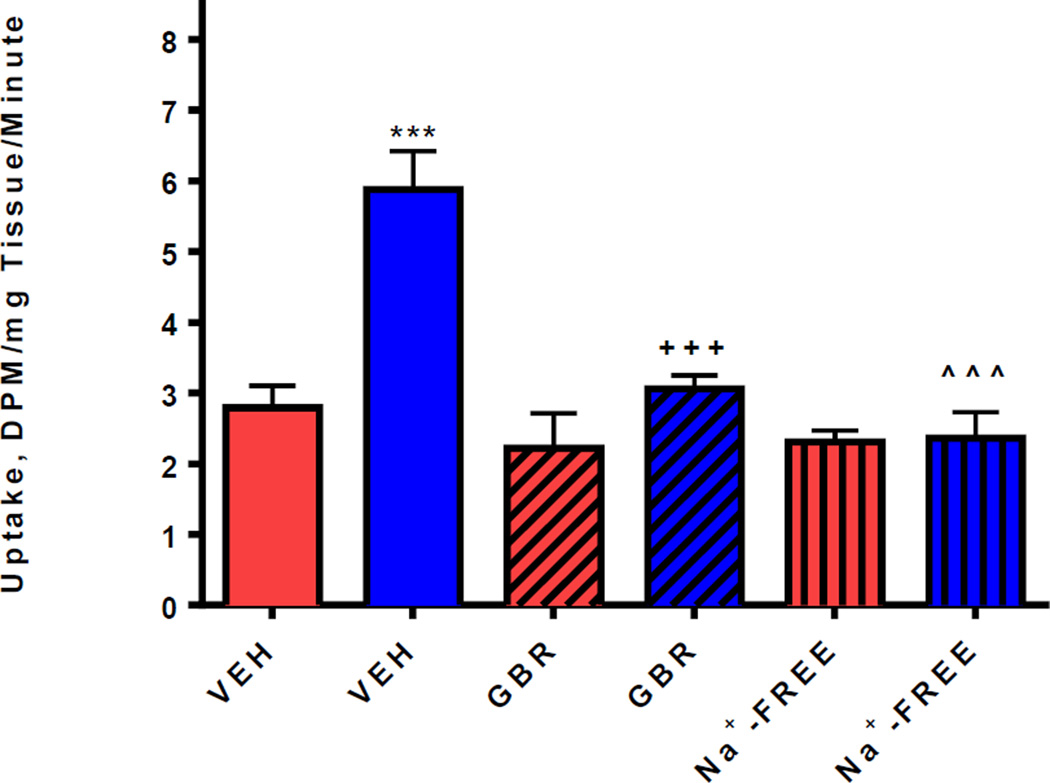
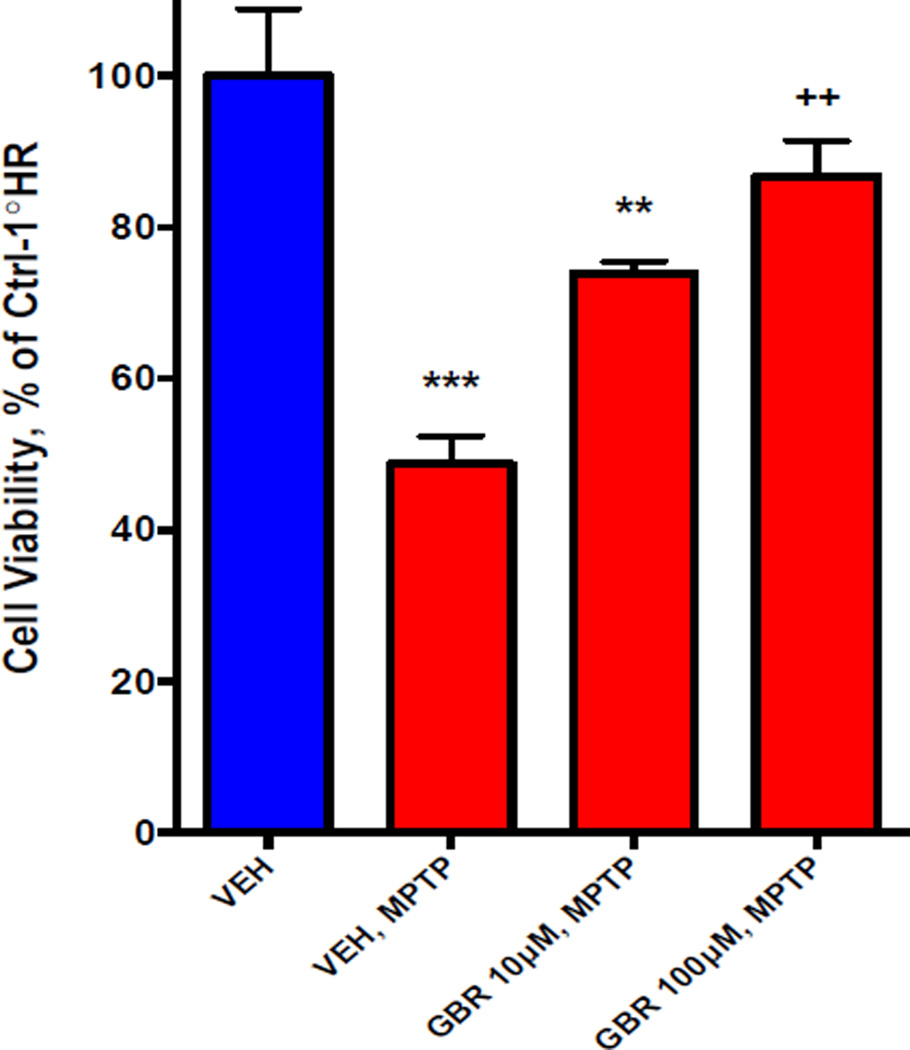
First, full-length cDNA of the human dopamine transporter (hDAT) was introduced into hairy root cultures of L. cardinalis by Agrobacterium rhizogenes-mediated transformation (Tsugawa et al 2004). The hDAT gene was present by PCR and its expression was demonstrated by gel-based electrophoresis. However for this selection procedure it is essential that the expressed protein should be functional, and retain the pharmacology of the native protein in mammalian systems. Therefore, the hDAT protein was shown to be expressed by specific binding of the DAT radioligand [3H]GBR12935 as for mammalian membranes (Reith & Selmeci 1992) (Figure 1). The transgenic hDAT plant cells showed significantly increased uptake of [3H]Dopamine (Williams & Steketee 2004), which was prevented by the DAT inhibitor GBR12909 (Rothman et al 2008) (Figure 2). Expression of the unmodified cDNA for the hDAT therefore appears to produce transgenic plant cells in which the functional hDAT is present in the plasma membrane”. In addition, neurotoxins that are concentrated intracellularly by activity of the DAT (Segura-Aguilar & Kostrzewa 2015) were more cytotoxic to transgenic hDAT plant cells than controls, and these were rescued by GBR12909 (see data for 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine, MPTP, in Figure 3). Exposure of transgenic hDAT plant cells to any of these neurotoxins should therefore favor survival of mutants overproducing endogenous inhibitors of the DAT (see Figure 4). 100uM MPP+, the ionic active moiety of MPTP, is rapidly lethal to non-mutant transgenic hDAT hairy roots and was chosen as the selection pressure.
Figure 1. Binding of [3H]GBR12935 to membranes derived from hairy root cultures of Lobelia cardinalis.
The upper curve shows saturable specific, one-site binding of [3H]GBR12935, a selective radioligand for the DAT, to membranes from transgenic hDAT hairy roots. Binding parameters were Kd 7.33 nM and Bmax= 1.04+0.9 pmol/mg protein (n=4)]. Non transgenic hairy roots (lower curve) showed no specific binding. Each data point represents mean±standard error of the mean (SEM) of 4 replicates and the curve represents best fit to a one-site binding model using Graphpad nonlinear regression software.
Figure 2. Uptake of [3H]Dopamine into hairy root cultures of L. cardinalis.
The first two bars compare [3H]Dopamine uptake into wildtype hairy roots (blue bars) with that into transgenic hairy roots (red bars). Cultures were incubated with [3H]Dopamine for 30min at 37°C, non-specific uptake was determined at 0°C. For each replicate, the radioactivity taken up by 10 roots was pooled and specific uptake calculated by subtracting non-specific uptake from total uptake values. Each bar represents four averaged replicates and data is expressed as mean±SEM DPM/mg tissue. The transgenic hDAT hairy roots showed significantly greater uptake of [3H]Dopamine (5.88±0.54^^^, One-way ANOVA, Bonferroni post hoc, P<0.001) than wildtype (2.79±0.31). Pretreatment with the selective inhibitor of the DAT, GBR12909 (100µM,+++, One-way ANOVA, Bonferroni post hoc, P<0.001), prevented the increased uptake of [3H]Dopamine into transgenic hDAT hairy roots, as did substitution of Na+-containing buffer with Na+-free buffer (***, One-way ANOVA, Bonferroni post hoc, P<0.001) (the function of the hDAT requires the presence of Na+), relative to vehicle-treated controls expressing hDAT(blue bar, VEH).
Figure 3. Increased toxicity induced by dopaminergic neurotoxins in transgenic (hDAT) hairy roots of L. cardinalis.
Toxicity in hairy roots was induced by addition of MPTP, MPP+ or 6-hydroxydopamine to the culture medium for 24h at concentrations that just failed to induce toxicity in wild-type hairy roots. Trypan blue staining was used to assess toxicity. Similar data were obtained for all these neurotoxins, in that toxicity was significantly increased in the transgenic hairy roots, relative to wild-type control. The data shown are means ± SEM of cell viability of hDAT transgenic hairy roots expressed as a percentage of wild type control, where n=6. 24h MPTP exposure caused toxicity in ~50% of cells in transgenic hDAT hairy roots (One way ANOVA followed by Bonferroni post hoc, p<0.001***). This toxicity was reduced by the selective DAT inhibitor GBR12909 at 10µM concentrations (p<0.01,** relative to vehicle treated groups) and MPTP toxicity was reversed by 100µM GBR12909 (p<0.01++, relative to MPTP treatment without GBR12909 pretreatment). These data indicate that cytotoxicity is increased by activity of the DAT, and that overproduction of DAT inhibitors should “rescue” transgenic (hDAT) hairy roots. However, MPTP requires intracellular metabolism to the active moiety, MPP+, so the cytotoxicity of MPP+ is more directly related to transport by the DAT. MPP+ (100µM) was therefore chosen for selection of mutant transgenic (hDAT) hairy roots.
2.2 Mutation, selection and analysis
Activation tagging mutagenesis (ATM) by A. rhizogenes (Fritze & Walden 1995) was used to insert viral “enhancer sequences” randomly into the genome of cultures derived from the stable transgenic hDAT line. This should cause stable activation of one or two plant genes at random in each mutant “secondary” hairy root derived from each transgenic (hDAT) “primary” hairy root. In normal medium, a single primary hairy root gives rise to ~20 secondary hairy roots, but when transferred to100uM MPP+ only ~1/300 transgenic secondary hairy roots develop, whereas all primary transgenic hDAT hairy roots die (see Figure 5). Toxin-resistant hairy roots were maintained in MPP+ for four months to ensure that the resistant phenotype was stable. The surviving cultures were then transferred to medium containing no MPP+ for two months to ensure that (a) the resulting pharmacological phenotype was stable (b) no MPP+ remained to confound pharmacological analysis (confirmed by GC/MS, see below). All toxin-resistant individuals (109 hairy roots) were sub-cultured, and extracts were analyzed for DAT inhibition (Wiliams & Steketee 2004) and lobinaline content in comparison with non-selected transgenic secondary hairy roots, and wild-type and transgenic hDAT primary hairy roots.
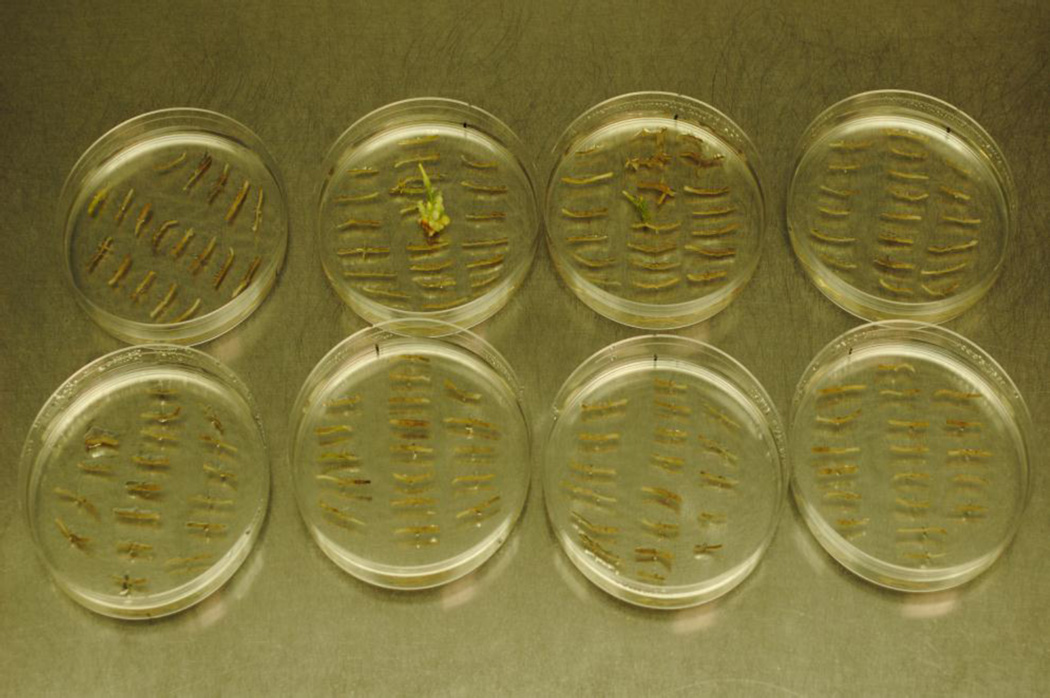
Figure 5. Representative transgenic hDAT hairy root cultures after activation tagging mutagenesis (ATM) in medium containing 100 µM MPP+.
This photograph shows about 120 primary hairy roots that have died under selection in 100 µM MPP+ with 2 mutant secondary hairy roots emerging from dead primary hairy roots, and developing normally (2nd and 3rd plates, upper row). In the absence of MPP+ about 2,000 secondary hairy roots would have developed from these primary hairy roots after ATM.
3. Results and Discussion
3.1 Pharmacological analysis
DAT inhibitory activity did not differ between the wild-type and transgenic hDAT primary hairy root cultures (Figure 6). Extracts from the non-selected secondary hairy roots showed a non-significant increase compared to the control populations, but extracts from the MPP+-resistant mutant population showed a markedly increased mean, and a much greater frequency of individuals with high levels of DAT inhibitory activity than any other population (Figure 6). Indeed 11 MPP+-resistant cultures showed such a high level of activity that they could not be accommodated on this scale (see Figure 6 legend). Overall, 58/109 individual toxin-resistant transgenic mutant cultures contained activity more than 3 SDs above the wild-type control mean. These changes probably result from stable mutations because none of the several thousand hairy roots that were not subjected to mutagenesis ever survived MPP+. Also, the DAT inhibitory phenotype persisted after 2 months in normal medium, when an elicited response should have subsided (Wang & Wu 2016). Silencing of the hDAT transgene is a possible alternative mechanism for MPP+-resistance, but the hDAT has remained functional in the transgenic line used for selection since this was generated 4 years ago. Thus, a single round of mutation and selection has driven the pharmacological phenotype of this species toward increased activity at the foreign target protein.
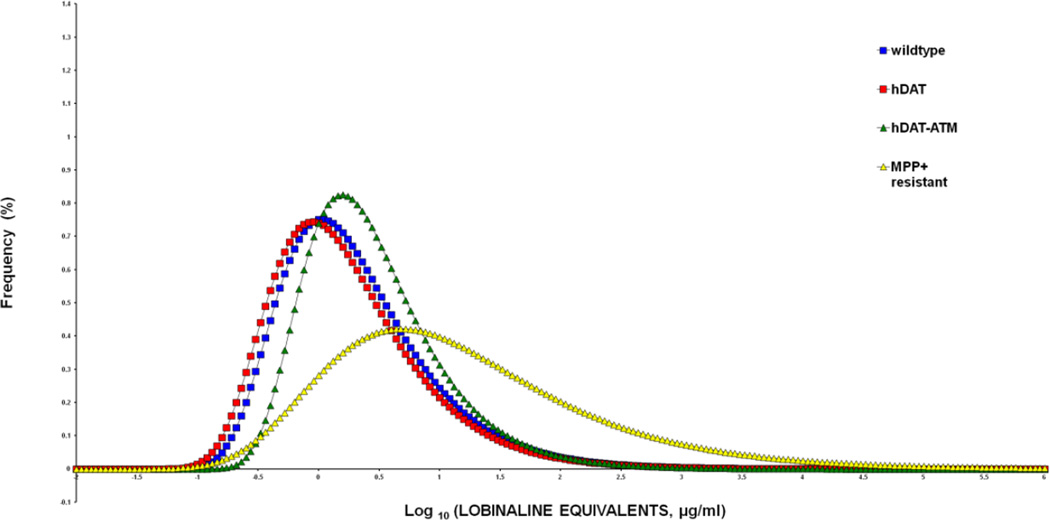
Figure 6. Frequency distribution of DAT inhibitory activity from individuals in different sub-populations of hairy root cultures of L. cardinalis.
Methanolic extracts from individual cultures were dried and re-suspended in DMSO/buffer before assay for inhibition of [3H]-Dopamine uptake into rat striatal synaptosomes. DAT inhibitory activity is expressed as log10 arbitrary units. For comparison with the data from Figure 3 these values were corrected to “lobinaline equivalents” based on lobinaline concentrations measured in methanol extracts of wild-type cultures. The means ± standard error of the mean (SEM) of each population (68–100 hairy roots in each) were then compared using one-way ANOVA followed by Bonferroni’s post-hoc analysis. The wildtype control (blue squares, 40.6±4.0 ug/ml), the transgenic hDAT cultures (red squares 32.8±2.7 ug/ml) and the non-selected transgenic hDAT mutant populations (green triangles 48.2±3.5 ug/ml) did not differ significantly from each other (P>0.05). However, the MPP+-resistant transgenic hDAT mutants (yellow triangles 282.1±52.5 ug/ml) showed significantly (P<0.0001) greater DAT inhibition than the other populations. This data and analysis does not include 11 MPP+-resistant mutants that completely prevented [3H]Dopamine uptake, and could not be accommodated on this scale.
3.2 Chemical analysis for lobinaline
GC/MS showed that the expression of the hDAT in primary hairy roots did not significantly affect the mean lobinaline content, or its distribution (Figure 7). The non-selected secondary hairy roots showed a small but significant increase in mean lobinaline content, as did the MPP+-resistant population. Although these two mutant populations did not differ in their means, the MPP+-resistant population showed a higher proportion of “lobinaline overproducers” (see legend to Figure 3) up to a maximum of ~20× the wild-type control mean in one mutant. These mutants continue to overproduce lobinaline in liquid culture, suggesting that they may be of value as production systems. However, only 16 mutants in this population contained sufficient lobinaline to explain the DAT inhibitory activity observed in the extract from the same culture. In addition, comparison of DAT inhibitory activity (Figure 2) with lobinaline content (Figure 3) shows a completely different distribution, and regression analysis shows no correlation between these measures. The MPP+-resistant population must therefore contain mutants in which increased DAT inhibition is caused by metabolites other than lobinaline.
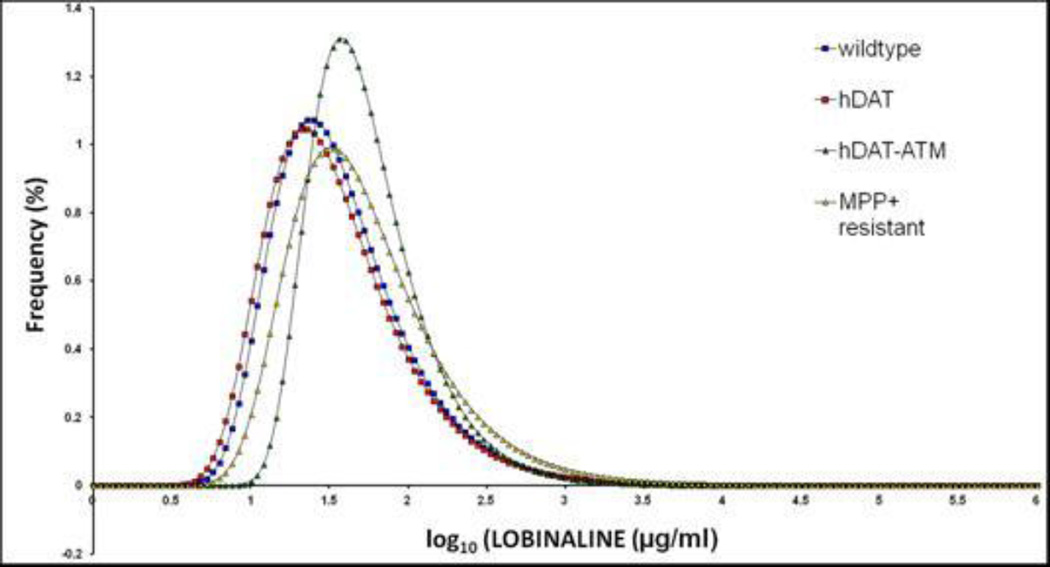
Figure 7. Frequency distribution of lobinaline concentrations in extracts from individuals in different sub-populations of hairy root cultures of L. cardinalis.
The Figure shows lobinaline concentration, determined by GC/MS in methanol extracts from individual cultures in the same populations shown in Figure 2. The same data presentation and statistical analysis was performed as for Figure 2. Means + SEM for lobinaline concentration are wildtype control (blue squares); 41.3±1.9 µg/ml; transgenic hDAT cultures (red squares) 38.3+1.8 µg/ml; non-selected transgenic hDAT ATM mutants (green triangles) 56.5+1.8µg/ml; MPP+-resistant transgenic hDAT mutants 59.2+2.5 µg/ml. One-way ANOVA followed by Bonferroni’s post-hoc analysis showed that extracts from the two mutant populations had a significantly greater lobinaline content from the control populations (P<0.001). The non-selected and MPP+-resistant mutants did not differ significantly from each other in means ± SEM, but the MPP+-resistant population included a higher proportion of individuals with a lobinaline concentration more than 3 SD above the control mean (37.9% vs 18.1%).
3.3 Chemical analysis for other protective metabolites
GC/MS identified 17 mutants with significantly increased concentrations of unsaturated fatty acids, which inhibit the DAT (Appell et al 2003). However, 25 mutants showed no increase in known DAT inhibitors, but contained “novel” GC peaks that are not observed in controls. Some probably represent lobinaline derivatives because they have similar retention times and molecular weights. Others contain complex alkaloids, with similarity to known synthetic DAT inhibitors (e.g. Kunstmann et al 1984), but the MS match is only partial. Methanol extracts from all these cultures were separated by preparative HPLC (Kesting et al 2009). Nine HPLC peaks were present that were not readily detectable in controls, and these were collected and assayed for DAT inhibition. All showed significant concentration-dependent inhibitory activity. This suggests the biosynthesis of novel metabolites with the desired effect on the foreign target protein. GC/MS analysis of the 51 resistant cultures that do not show increased DAT inhibitory activity indicates that many are over-producing metabolites that inhibit MPP+-induced oxidative damage to mitochondria (Cassarino et al 1999). These include squalene (Kabuto et al 2013) and 2,4-Di-tert-butylpheno (Choi et al 3013). Many of these cultures also have novel GC/MS peaks, which may represent novel neuroprotective metabolites (Xu & Moller 2011)
4. Conclusion
In these proof-of-concept studies we show that a single round of mutation and selection can rapidly “evolve” plant cells toward a specific pharmacological phenotype based on interactions with a foreign target protein. The same technology could be applied to agrochemical and nutritional targets, with major implications for agriculture, but our focus is on the pharmaceutical industry. Here the technology could be developed for plant drug discovery and production, as well as to optimize biosynthesis in medicinal plants to enhance therapeutic activity in traditional medicines. It should be possible to protect intellectual property in all of these products because they are derived from unique genetic mutations. If we can obtain proof of application, the technology could make plants once again a major global source of medicines, a position they have occupied throughout most of human history.
Highlights.
We discovered a novel inhibitor of dopamine transport in Lobelia cardinalis
We functionally expressed the Human dopamine transporter in L. cardinalis plant cells
Next a Gain-of-function mutant transgenic population was generated from these cells, in presence of a neurotoxin accumulated by the transporter.
The resulting toxin-resistant population was highly enriched in mutants with increased inhibitory activity on the transporter
Some toxin-resistant mutants overproduce the known active metabolite from wild-type.
Many others contain other active metabolites including some not readily detectable in the wild-type plant
Acknowledgments
This research was supported by SBIR R44AA018226 “Selection-driven plant metabolites for treatment of CNS diseases” awarded under the Roadmap initiative “Novel tools for brain and behavior” to Naprogenix Inc (PI JML) from the National Institute for Alcohol Abuse and Alcoholism. The authors greatly acknowledge the technical expertise provided by Mr. Jatinder Sambi, and Ms Grace Burgess-Poole. Conflict of interest: DTR and JML are employees of Naprogenix Inc and JML also owns stock in the company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Appell M, Berfield JL, Reith ME. Inhibition by arachidonic acid and other fatty acids of dopamine uptake at the human dopamine transporter. Eur J Pharmacol. 2003 Oct 8;478(2–3):89–95. doi: 10.1016/j.ejphar.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Brown DP, Rogers DT, Pomerlau F, Siripurapu KB, Kulshrestha M, Gerhardt GA, Littleton JM. Novel multifunctional pharmacology of lobinaline, the major alkaloid from Lobelia cardinalis. Fitoterapia. 2016 doi: 10.1016/j.fitote.2016.04.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassarino DS, Parks JK, Parker WD, Bennett JP. The Parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1999;1453(1):49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Chen R, Wu X, Wei H, Han DD, Gu HH. Molecular cloning and functional characterization of the dopamine transporter from Eloria noyesi, a caterpillar pest of cocaine-rich coca plants. Gene. 2006 Jan 17;366(1):152–160. doi: 10.1016/j.gene.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Kim JK, Kim HK, Harris K, Kim CJ, Park GG, Park CS, Shin DH. 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J Med Food. 2013 Nov;16(11):977–983. doi: 10.1089/jmf.2012.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukhanina EV, Apuya NR, Yoo HD, Wu CY, Davidow P, Krueger S, Flavell RB, Hamilton R, Bobzin SC. Expression of human nuclear receptors in plants for the discovery of plant-derived ligands. J Biomol Screen. 2007;12:385–395. doi: 10.1177/1087057107299255. [DOI] [PubMed] [Google Scholar]
- Fritze K, Walden R. Gene activation by T-DNA tagging. Methods Mol Biol. 1995;44:281–294. doi: 10.1385/0-89603-302-3:281. [DOI] [PubMed] [Google Scholar]
- Gunjan SK, Rogers DT, Zhang J, Yun KY, Falcone DL, Littleton J. Use of alpha-, beta-Estrogen Receptor as a new tool for detection of specific small molecule activity. Plant Mol Biol Report. 2015 Dec 1;33(6):1837–1843. doi: 10.1007/s11105-015-0879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howat S, Park B, Oh IS, Jin YW, Lee EK, Loake GJ. Paclitaxel: biosynthesis, production and future prospects. N Biotechnol. 2014 May 25;31(3):242–245. doi: 10.1016/j.nbt.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Kabuto H, Yamanushi TT, Janjua N, Takayama F, Mankura M. Effects of squalene/squalane on dopamine levels, antioxidant enzyme activity, and fatty acid composition in the striatum of Parkinson's disease mouse model. J Oleo Sci. 2013;62(1):21–28. doi: 10.5650/jos.62.21. [DOI] [PubMed] [Google Scholar]
- Kesting JR, Tolderlund IL, Pedersen AF, Witt M, Jaroszewski JW, Staerk D. Piperidine and tetrahydropyridine alkaloids from Lobelia siphilitica and Hippobroma longiflora. J Nat Prod. 2009 Feb 27;72(2):312–315. doi: 10.1021/np800743w. [DOI] [PubMed] [Google Scholar]
- Kunstmann R, Lerch U, Gerhards H, Leven M, Schacht U. 2,3,4,4a,5,9b-Hexahydro-1H-indeno[1,2-b]pyridines: potential antidepressants. J Med Chem. 1984 Apr;27(4):432–439. doi: 10.1021/jm00370a004. [DOI] [PubMed] [Google Scholar]
- Littleton JM. The future of plant drug discovery. Expert Opinion on drug discovery. 2007;1:673–683. doi: 10.1517/17460441.2.5.673. [DOI] [PubMed] [Google Scholar]
- Littleton J, Rogers T, Falcone D. Novel approaches to plant drug discovery based on high throughput pharmacological screening and genetic manipulation. Life Sci. 2005 Dec 22;78(5):467–475. doi: 10.1016/j.lfs.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Manske RHF. Lobinaline, an alkaloid from Lobelia cardinalis L. Can. J. Research. 1938;16 B:445–448. [Google Scholar]
- Reith ME, Selmeci G. Radiolabeling of dopamine uptake sites in mouse striatum: comparison of binding sites for cocaine, mazindol, and GBR 12935. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):309–318. doi: 10.1007/BF00168692. [DOI] [PubMed] [Google Scholar]
- Robison MM, Pierson WG, et al. Structure of lobinaline. Tetrahedron Lett. 1964;23–24:1513.28–1519.28. [Google Scholar]
- Rogers DT, Falcone DL, Littleton JM. A functional genomics strategy for plant drug discovery. Pharmagenomics. 2003;3:27–34. [Google Scholar]
- Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008 Jan 1;75(1):2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Krämer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014 Jan;466(1):25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J1, Kostrzewa RM. Neurotoxin Mechanisms and Processes Relevant to Parkinson's Disease: An Update. Neurotox Res. 2015 Jan 29; doi: 10.1007/s12640-015-9519-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tsugawa H, Kagami T, Suzuki M. High-frequency transformation of Lobelia erinus L. by Agrobacterium-mediated gene transfer. Plant Cell Rep. 2004 May;22(10):759–764. doi: 10.1007/s00299-004-0758-2. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wu JY. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. Adv Biochem Eng Biotechnol. 2013;134:55–89. doi: 10.1007/10_2013_183. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Characterization of dopamine transport in crude synaptosomes prepared from rat medial prefrontal cortex. J Neurosci Methods. 2004 Aug 30;137(2):161–165. doi: 10.1016/j.jneumeth.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Xu XM, Moller SG. The value of Arabidopsis research in understanding human disease states. Curr Opin Biotechnol. 2011;22(2):300–307. doi: 10.1016/j.copbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zeng Y, Kermode AR. A plant cell-based system that predicts aβ42 misfolding: Potential as a drug discovery tool for Alzheimer's disease. Mol Genet Metab. 2012 Nov;107(3):571–579. doi: 10.1016/j.ymgme.2012.08.010. [DOI] [PubMed] [Google Scholar]