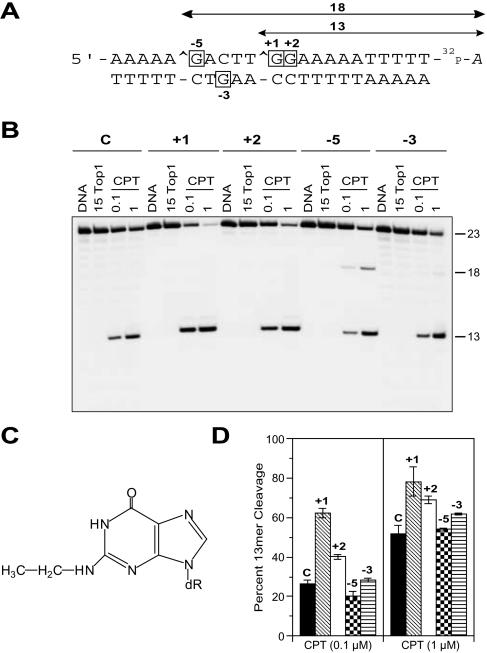
Figure 1.
Enhancement of CPT-induced Top1-mediated DNA cleavage by N2-ethyl-dG adducts at specific positions. (A) Sequence of the 22mer oligonucleotide used for this study with the 3′ radiolabel (cordycepin indicated by 32P-A) on the scissile strand of the duplex. Algebraic numbers represent the four modified oligonucleotides studied, where the numbered dG is adducted to an ethyl group (C). The modified bases corresponding to these positions within the oligonucleotides are indicated as boxed bases. The Top1-mediated DNA cleavage site is indicated by a caret (∧). (B) The control (C) oligonucleotide was reacted for 15 min at 25°C with Top1 in the absence of drug (15 Top1) or in the presence of 0.1 or 1 μM CPT (CPT). Similar reactions were carried out using each of the four adducted (+1, +2, −5 and −3) duplex oligonucleotides. Numbers 23, 18 and 13 indicate the size of the 3′-labeled oligonucleotide and the Top1-mediated DNA fragments, respectively. (C) Chemical structure of the N2-ethyl-dG adduct. (D) Quantitation of the Top1-mediated cleavage products obtained with CPT (0.1 and 1 μM) (mean ± SD of three independent experiments).