Figure 1.
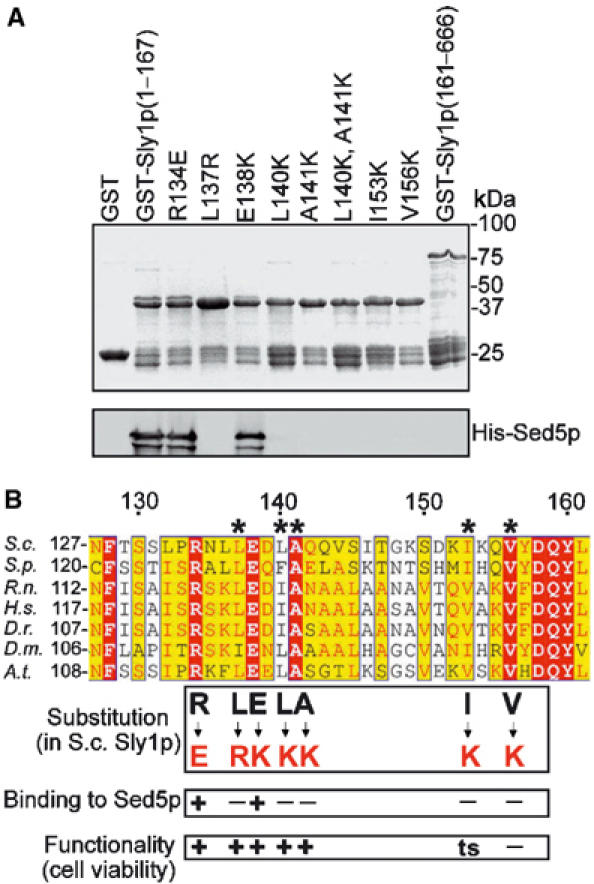
Sly1p mutants unable to bind to Sed5p are functional. (A) Fragments of wild-type and mutant Sly1 protein fused to GST were bound to glutathione–Sepharose and incubated with His-tagged Sed5p (0.2 μg/μl) purified from Escherichia coli. After extensive washing, the beads were mixed with SDS sample buffer, boiled and bound proteins were separated by SDS–PAGE and viewed by Coomassie blue staining (upper panel) or immunoblotting with affinity-purified anti-Sed5p antibodies (lower panel). (B) Sly1p sequences from different species were aligned by a Clustalw software. Conserved amino acids are highlighted by different colors. The five amino acids that form the hydrophobic Sed5-binding pocket are designated by asterisks. Amino-acid substitutions and their consequences are indicated.
