Figure 6.
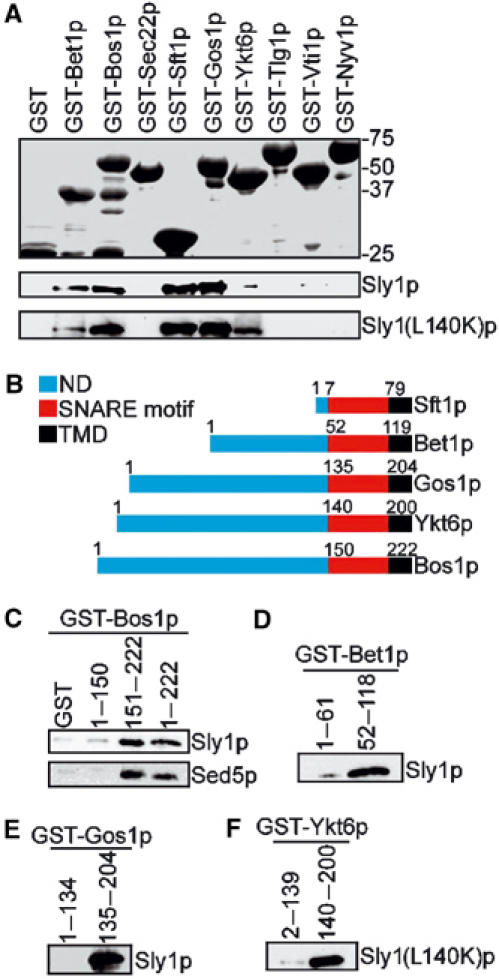
Sly1p directly binds to v-SNAREs and nonsyntaxin t-SNAREs. (A) Bacterially produced nonsyntaxin SNAREs of various protein transport steps fused to GST were immobilized on glutathione–Sepharose beads and incubated with purified Sly1p (0.2 μg/μl) or Sly1(L140K)p (0.2 μg/μl). Proteins bound on beads were analyzed by SDS–PAGE and Coomassie blue staining (upper panel) or by immunoblotting using anti-Sly1p antibodies (lower panel). (B) Domain structures of nonsyntaxin SNAREs Bet1p, Bos1p, Sft1p, Gos1p and Ykt6p. ND, N-terminal domain; TMD, transmembrane domain. (C–F) The N-terminal region or the SNARE motifs of different SNAREs fused to GST and bound to glutathione–Sepharose beads were incubated with Sly1p (0.2 μg/μl) or Sly1(L140K)p (0.2 μg/μl). GST fusions and bound proteins were analyzed by immunoblotting. In (B), the binding of Bos1p fragments to Sed5p (0.2 μg/μl) is shown.
