Figure 1.
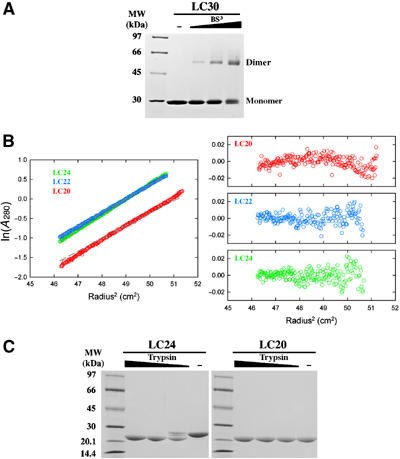
Dimerization domain of E. coli MutL. (A) A Coomassie-blue-stained SDS gel of LC30 dimers crosslinked by 0.11, 0.33 and 1 mM BS3. (B) Sedimentation equilibrium analysis of LC24, LC22 and LC20. Profiles of the three proteins (color coded) at 12 000 r.p.m. and 4°C are plotted on the left. The gray dashed lines represent expected values for the corresponding dimers. Residuals for each protein are shown on the right. (C) Trypsin digestion of LC24 and LC20. In all, 9 μl of 0.5 mg/ml LC24 or LC20 was digested by 1 μl of 0.01, 0.03 or 0.1 mg/ml trypsin in the protein storage buffer for 1 h at 22°C. The digestion products were separated by SDS–PAGE and stained with Coomassie blue.
