Abstract
Objective(s):
Alzheimer’s disease (AD) as progressive cognitive decline and the most common form of dementia is due to degeneration of the cholinergic neurons in the brain. Therefore, administration of the acetylcholinesterase (AChE) inhibitors such as donepezil is the first choice for treatment of the AD. In the present study, we focused on the synthesis and anti-cholinesterase evaluation of new donepezil like analogs.
Materials and Methods:
A new series of phthalimide derivatives (compounds 4a-4j) were synthesized via Gabriel protocol and subsequently amidation reaction was performed using various benzoic acid derivatives. Then, the corresponding anti-acetylcholinesterase activity of the prepared derivatives (4a-4j) was assessed by utilization of the Ellman’s test and obtained results were compared to donepezil. Besides, docking study was also carried out to explore the likely in silico binding interactions.
Results:
According to the obtained results, electron withdrawing groups (Cl, F) at position 3 and an electron donating group (methoxy) at position 4 of the phenyl ring enhanced the acetylcholinesterase inhibitory activity. Compound 4e (m-Fluoro, IC50 = 7.1 nM) and 4i (p-Methoxy, IC50 = 20.3 nM) were the most active compounds in this series and exerted superior potency than donepezil (410 nM). Moreover, a similar binding mode was observed in silico for all ligands in superimposition state with donepezil into the active site of acetylcholinesterase.
Conclusion:
Studied compounds could be potential leads for discovery of novel anti-Alzheimer agents in the future.
Keywords: Acetylcholinesterase Alzheimer, Docking, Ellman test, Phthalimide, Synthesis
Introduction
Alzheimer’s disease (AD), discovered by Dr Alois Alzheimer in 1907, is described as a degenerative disease of the central nervous system (CNS) characterized especially by premature senile mental deterioration. AD patients exhibit marked decline in cognitive ability and severe behavioral abnormalities such as irritability, anxiety, depression, disorientation, and restlessness (1). The disease is characterized by the appearance of senile plaques mainly composed of amyloid β (Aβ), and by the development of neurofibrillary tangles in patients’ brain (2). AD is associated with certain neurological changes such as selective loss of cholinergic neurons in the forebrain, extracellular deposition of amyloid peptide, accumu-lation of intracellular neurofibrillary tangles, and disordered cognitive functions. It was recently reported that in a significant proportion of AD patients there is also a slowed motor activity and extrapyra-midal dysfunction resembling that seen in Parkinson’s disease (PD) (3).
Treatment for AD is a daily challenge for physicians and pharmacists. The cognitive symptoms that characterize Alzheimer’s disease are thought to be related to the degeneration of cholinergic neurons in the cerebral cortex and subcortical structures. A number of preclinical studies have suggested an association between the cholinergic system and cognition. For example, experimental lesions of the basal forebrain cholinergic system and treatment of animals with muscarinic antagonists produce memory deficits (4, 5). There has been substantial progress in the therapeutic approach to AD in the past few years (Figure 1). Among different strategies investigated to improve cholinergic neurotransmission, the reduction of ACh hydrolysis by AChE inhibitors is up to date the prevalent effective AD symptomatic treatment (6, 7).
Figure 1.
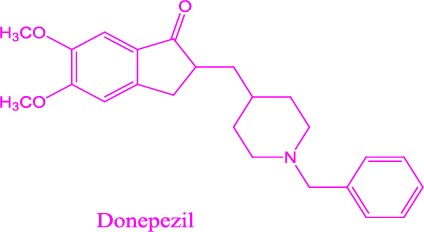
Structures of acetycholinesterase inhibitors in the market
Recently, some (8-10) reports about the efficacy of phthalimide derivatives in inhibition of AChE have been presented (Figure 2). Hence, in the present study, we focused on the design and synthesis of new anti-acetylcholinesterase agents with phthalimide-based structure. In fact, phthalimide based compounds have similar pharmacophoric portions like indanone ring of the donepezil and are able to act as peripheral binding site inhibitor of AChE (8-14).
Figure 2.
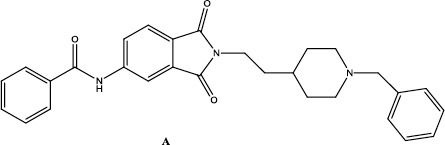
Structure of a phthalimide based compound as acetylcholinesterase inhibitor
Scheme 1.

Synthetic pathway for preparation of 4a-4j
Materials and Methods
Chemistry
All chemicals consisting starter materials, reagents and solvents were purchased from commercial vendors such as Merck and Sigma-Aldrich. The purity of the synthesized compounds was confirmed by thin layer chromatography (TLC) using various solvents of different polarities. Merck silica gel 60 F254 plates were applied for analytical TLC. Column chromatography was performed on Merck silica gel (70-230 mesh) to purify the intermediate and final compounds. 1H-NMR and 13CNMR spectra were recorded using a Bruker 400 and 250 MHz spectrometers respectively, and chemical shifts are expressed as δ (ppm) with tetramethylsilane (TMS) as internal standard. Intended compounds were dissolved in deutrated chloroform for NMR spectra acquisition. The IR spectra were obtained on a Shimadzu 470 spectrophotometer (potassium bromide disks). Melting points were determined electrothermal melting point analyzer and are uncorrected. The mass spectra were run on a Finigan TSQ-70 spectrometer (Finigan, USA) at 70 eV. Elemental analyses were carried out on a CHNO rapid elemental analyzer (GmbH-Germany) for C, H, N, and O and the results are within ± 0.4% of the theoretical values.
Synthesis of 2-(2-(Piperazin-1-yl)ethyl)isoindoline-1,3-dione (3)
In a flat bottom flask, equimolar quantities of phthalic anhydride, N-amnioethylpiperazine and triethylamine (Et3N) were mixed in appropriate amount of toluene solvent. The reaction mixture was refluxed for 24 hr and the termination of the reaction and formation of the desired product was confirmed using TLC. The discolor-ration of the reaction medium and formation of a yellow precipitate was also an indicator of the progress of the reaction. Then, toluene was evaporated under reduced pressure using rotary evaporator apparatus and the obtained yellow viscose and oily residue was washed several times by ethyl acetate (EtOAc) and diethyl ether (Et2O) (9).
General procedure for synthesis of compounds 4a-4j:
In a flat bottom flask, equimolar quantities of appropriate benzoic acid derivative, N-ethyl-N-dimethylaminopropyl carbodiimde (EDC) and HOBt were stirred in a acetonitrile solvent for 30 min. Then, equimolar quantity of compound 3 was added and stirring was continued for 24 hr. The product formation was proved using TLC and therefore the end of the reaction was determined. Acetonitrile was evaporated using rotary evaporator apparatus and the residue was dissolved in ethyl acetate/water (50:50). The mixture was moved to the separatory extraction funnel and the aqueous phase was removed. The organic phase was extracted two times by sodium bicarbonate 5% and brine. Anhydrous sodium sulfate was added for drying and then filtered. The ethyl acetate was evaporated under reduced pressure and the obtained oily residue was treated by methanolic HCl to form the hydrochloride salt of the corresponding base for each compound (10, 15-19).
Preliminary design
According to the literature, there is three distinct parts in the structure of donepezil that are necessary for anticholinesterase activity (Figure 3) (1, 12). Namely, phenyl ring of the indanone moiety (part A), nitrogen of the piperidine ring (part B) and phenyl ring of the benzyl group (part C) are the critical parts for interacting with the corresponding amino acids in the active site of acetyl cholinesterase enzyme. Phenyl ring of the indanone moiety participates in a π-π stacking interaction with Trp 279. Nitrogen of the piperidine ring is important for creating a cation-π interaction with Phe 330. There is another π-π stacking interaction between phenyl ring of the benzyl moiety of the donepezil and indanone ring of the Trp 84. Hence, according to the pharmacophoric necessities that mentioned above for donepezil interaction, a new series of phthalimide based derivatives were designed (Figure 3).
Figure 3.
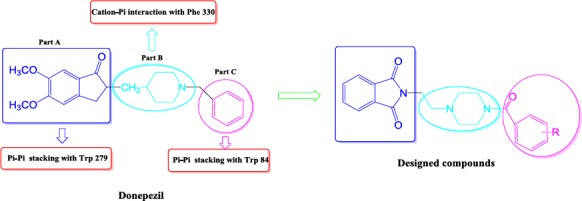
Design of compounds 4a-4j according to the pharmacophoric necessities of donepezil
Docking
The study of molecular docking of all ligands was performed using ArgusLab 4.0 software [20). All intended ligands were built in arguslab workspace and energy minimization was performed using AM1 as semiemperical method for all ligands. The pdb files of acetyl cholinesterase in complex with donepezil (pdb code: 1EVE) was downloaded from brookhaven protein databank (21, 22). The geometry optimization of the protein structure was optimized geometrically by universal force field (UFF) as a molecular mechanic method. The docking process was done for all ligands in the workspace of ArgusLab software after defining the related groups for each ligand as well as protein. The binding site of donepezil was defined as binding site for searching the best pose and conformation for all ligands. Binding mode and related interactions of ligands with acetyl cholinesterase enzyme were explored in Molegro molecular viewer software (23).
Pharmacology
Ellman test was applied to investigate the capability of the synthesized compounds toward the inhibition of the acetylcholinesterase enzyme. Lyophilized powder of acetylcholinesterase from electric eel source (AChE, E.C. 3.1.1.7, Type V-S, 1000 unit) was purchased from Sigma-Aldrich (Steinheim, Germany). 5,5’-Dithiobis-(2-nitrobenzoic acid, DTNB), potassium dihydrogen phosphate (KH2PO4), dipotassium hydrogen phosphate (K2HPO4), potassium hydroxide (KOH), sodium hydrogen carbonate (NaHCO3), and acetylthiocholine iodide were purchased from Fluka (Buchs, Switzerland). Spectrophotometric measurements were run on a Cecil BioAquarius CE 7250 Double Beam Spectrophotometer.
Compounds 4a-4j were dissolved in a mixture of 20 ml distilled water and 5 ml methanol and then diluted in 0.1 M KH2PO4/K2HPO4 buffer (pH 8.0) to yield a final concentration range. According to the literature, the Ellman test was performed for assessment of the anticholinesterase activity of intended compounds in vitro. To achieve 20-80% inhibition of AChE activity five different concentrations of each compound were tested. Compounds 4a-4j were added to the assay solution and preincubated at 25 °C with the enzyme for 15 min followed by adding 0.075 M of acetylthiocholine iodide. After rapid and immediate mixing the change of absorption was measured at 412 nm.
The blank reading contained 3 ml buffer, 200 µl water, 100 µl DTNB and 20 µl substrate. The reaction rates were calculated, and the percent inhibition of test compounds was determined. Each concentration was analyzed in triplicate, and IC50 values were determined graphically from inhibition curves (log inhibitor concentration vs percent of inhibition) (24, 25).
Results
Chemistry
All synthesized derivatives were characterized by spectroscopic methods such as 1H-NMR, IR and MS. Melting points were also measured and obtained yields were calculated (Table 1).
Table 1.
Properties of compounds 4a-4j
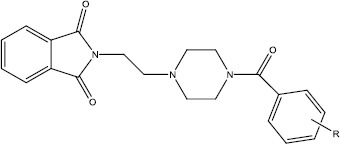 | |||||
|---|---|---|---|---|---|
| Compound | (R) | Yield (%) | mp (°C) | MW (g/mol) | Chemical formula |
| 3 | - | 61 | 105-109 | 259 | C14H17N3O2 |
| 4a | 2-Cl | 23 | 229 | 397 | C21H20N3O3Cl |
| 4b | 3-Cl | 18 | 203 | 397 | C21H20N3O3Cl |
| 4c | 4-Cl | 15 | 229 | 397 | C21H20N3O3Cl |
| 4d | 2-F | 35 | 247 | 381 | C21H20FN3O3 |
| 4e | 3-F | 13 | 243 | 381 | C21H20FN3O3 |
| 4f | 4-F | 52 | 229 | 381 | C21H20FN3O3 |
| 4g | 2-OCH3 | 14 | 211 | 393 | C23H23N3O4 |
| 4h | 3-OCH3 | 30 | 127 | 393 | C23H23N3O4 |
| 4i | 4-OCH3 | 36 | 127 | 393 | C23H23N3O4 |
| 4j | 2-NO2 | 49 | 135 | 408 | C21H23N4O5 |
2-(2-(Piperazin-1-yl) ethyl) isoindoline-1,3-dione (3)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.37 (m, piperazine), 2.54 (m, piperazine), 3.22 (t, phthalimide-CH2-CH2-piperazine), 3.44 (t, phthalimide-CH2-CH2-piperazine), 4.73 (NH, piperazine), 7.35-7.85 (m, 4H, phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 34.8 (Phthalimide-C-C-Piperazine), 36.0 (C3,5-Pyridine), 44.7 (Phthalimide-C-C-Piperazine), 52.8 (C2,6-Pyridine), 123.0 (C4,7-Phthalimide), 130.3 (C3a,7a-Phthalimide), 134.4 (C5,6-Phthalimide), 168.3 (C=O, Phthalimide). IR (KBr, cm-1) ῡ: 3380 (N-H, Stretch), 3330, 3157 (C-H, Aromatic), 3111 (C-H, Aromatic), 2924 (C-H, Stretch, Asymmetric, Aliphatic), 1730 (C=O, Stretch), 1681, 1521 (N-H, Bending), 1489 (C=C, Aromatic), 1458, 1328, 1303, 1186 (C-N, Stretch), 1143, 1035, 910, 750, 710. MS (m/z, %): 259 (M+, 10), 224 (30), 174 (30), 160 (60), 149 (85), 99 (100), 70 (70), 57 (65), 41 (40).
2-(2-(4-(2-Chlorobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4a)
1H NMR (CDCl3, 400 MHz) δ (ppm): 1.72 (brs, 4H, Piperazine), 2.98 (brs, 4H, Piperazine), 3.42 (m, 2H, Phthalimide-CH2-CH2-Piperazine), 4.20 (m, 2H, Phthalimide-CH2-CH2-Piperazine), 7.36-7.46 (m, 4H, 2-Chlorophenyl), 7.78 (q, 2H, H5,6-Phthalimide), 7.90 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 32.1 (Phthalimide-C-C-Piperazine), 37.8 (C3-Piperazine), 42.8 (C5-Piperazine), 50.4 (Phthalimide-C-C-Piperazine), 53.2 (C2,6-Piperazine), 123.3 (C4,7-Phthalimide), 126.9 (C5-2-Chlorophenyl), 127.2 (C6-4-Chlorophenyl), 127.8 (C4-2-Chlorophenyl), 129.2 (C3-2-Chlorophenyl), 129.6 (C1-2-Chlorophenyl), 131.1 (C3a,7a-Phthalimide), 132.1 (C5,6-Phthalimide), 134.5 (C2-2-Chlorophenyl), 165.7 (C=O, Phthalimide), 168.0 (C=O, 2-Chlorobenzoyl). IR (KBr, cm-1) ῡ: 3095 (C-H, Stretch, Aromatic), 2924 (C-H, Stretch, Asymmetric, Aliphatic), 2858 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1639 (C=O, Stretch, Amide), 1465, 1246 (C-N, Stretch).
2-(2-(4-(3-Chlorobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4b)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.91 (brs, 4H, Piperazine), 3.41 (brs, 4H, Piperazine), 3.88 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 7.43-7.49 (m, 4H, 3-Chlorophenyl), 7.78 (q, 2H, H5,6-Phthalimide), 7.91 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 35.8 (Phthalimide-C-C-Piperazine), 54.2 (C3-Piperazine), 45.9 (C5-Piperazine), 52.7 (Phthalimide-C-C-Piperazine), 55.3 (C2,6-Piperazine), 124.6 (C4,7-Phthalimide), 127.1 (C6-3-Chlorophenyl), 129.7 (C5-3-Chlorophenyl), 131.8 (C2-3-Chlorophenyl), 133.1 (C4-3-Chlorophenyl), 134.9 (C3-3-Chlorophenyl), 135.2 (C5,6-Phthalimide), 136.9 (C1-3-Chlorophenyl), 166.2 (C=O, Phthalimide), 169.5 (C=O, 3-Chlorobenzoyl). IR (KBr, cm-1) ῡ: 3066 (C-H, Stretch, Aromatic), 2924 (C-H, Stretch, Asymmetric, Aliphatic), 2854 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1631 (C=O, Stretch, Amide), 1249 (C-N, Stretch).
2-(2-(4-(4-Chlorobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4c)
1H NMR (CDCl3, 400 MHz) δ (ppm): 1.78 (brs, 4H, Piperazine), 2.91 (brs, 4H, Piperazine), 3.42 (m, 2H, Phthalimide-CH2-CH2-Piperazine), 4.20 (m, 2H, Phthalimide-CH2-CH2-Piperazine), 7.43 (m, 4H, 4-Chlorophenyl), 7.78 (q, 2H, H5,6-Phthalimide), 7.89 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 32.0 (Phthalimide-C-C-Piperazine), 37.3 (C3-Piperazine), 38.03 (C5-Piperazine), 50.4 (Phthalimide-C-C-Piperazine), 53.1 (C2,6-Piperazine), 123.1 (C4,7-Phthalimide), 128.3 (C3,5-4-Chlorophenyl), 128.6 (C2,6-4-Chlorophenyl), 129.2 (C1-4-Chlorophenyl), 131.9 (C3a,7a-Phthalimide), 133.5 (C5,6-Phthalimide), 134.4 (C4-4-Chlorophenyl), 167.9 (C=O, Phthalimide), 168.1 (C=O, 4-Chlorobenzoyl). IR (KBr, cm-1) ῡ: 3097 (C-H, Stretch, Aromatic), 2962 (C-H, Stretch, Asymmetric, Aliphatic), 2924 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1635 (C=O, Stretch, Amide), 1288 (C-N, Stretch).
2-(2-(4-(2-Fluorobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4d)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.98 (brs, 4H, Piperazine), 3.46 (brs, 4H, Piperazine), 3.69-4.21 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 7.12-7.55 (m, 4H, 2-Fluorophenyl), 7.77 (q, 2H, H5,6-Phthalimide), 7.88 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 34.6 (Phthalimide-C-C-Piperazine), 42.2 (C3-Piperazine), 41.1 (C5-Piperazine), 50.9 (Phthalimide-C-C-Piperazine), 53.6 (C2,6-Piperazine), 116.2 (d, C3-2-Fluorophenyl), 120.2 (d, C1-2-Fluorophenyl), 123.8 (C4,7-Phthalimide), 125.6 (C5-2-Fluorophenyl), 127.5 (C6-2-Fluorophenyl), 130.6 (C3a,7a-Phthalimide), 132.1 (C4-2-Fluorophenyl), 136.1 (C5,6-Phthalimide), 161.1 (d, C2-2-Fluorophenyl), 166.2 (d, C=O, 2-Fluorobenzoyl), 167.3 (C=O, Phthalimide). IR (KBr, cm-1) ῡ: 3132 (C-H, Stretch, Aromatic), 2954 (C-H, Stretch, Asymmetric, Aliphatic), 2927 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1650 (C=O, Stretch, Amide), 1608 (C=C, Stretch, Aromatic), 1470, 1253 (C-N, Stretch). MS (m/z, %): 381 (M+, 5), 221 (100), 166 (10), 123 (50), 95 (10), 75 (3), 56 (3).
2-(2-(4-(3-Fluorobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4e)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.91 (brs, 4H, Piperazine), 3.44 (brs, 4H, Piperazine), 3.63-4.21 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 7.22 (m, 2H, H5,6-3-Fluorophenyl), 7.45 (m, 1H, H4-3-Fluorophenyl), 7.73 (m, 1H, H2-3-Fluorophenyl), 7.77 (q, 2H, H5,6-Phthalimide), 7.89 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 37.3 (Phthalimide-C-C-Piperazine), 44.6 (C3-Piperazine), 45.5 (C5-Piperazine), 53.1 (Phthalimide-C-C-Piperazine), 55.6 (C2,6-Piperazine), 114.5 (d, C2-3-Fluorophenyl), 119.2 (d, C4-3-Fluorophenyl), 122.4 (C4,7-Phthalimide), 125.2 (C6-3-Fluorophenyl), 130.5 (d, C5-3-Fluorophenyl), 132.6 (C3a,7a-3-Fluorophenyl), 133.3 (d, C1-3-Fluorophenyl), 136.4 (C5,6-Phthalimide), 159.2 (C3-3-Fluorophenyl), 167.2 (C=O, Phthlimide), 168.4 (d, C1-2-Fluorophenyl).
2-(2-(4-(4-Fluorobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4f)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.07 (brs, 4H, Piperazine), 2.96 (brs, 4H, Piperazine), 3.44-4.20 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 7.15 (m, 2H, 4-Fluorophenyl), 7.49 (m, 2H, 4-Fluorophenyl), 7.77 (q, 2H, H5,6-Phthalimide), 7.88 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 32.0 (Phthalimide-C-C-Piperazine), 50.6 (Phthalimide-C-C-Piperazine), 53.1 (C2,6-Piperazine), 115.5 (d, C3,5-4-Fluorophenyl), 123.1 (C4,7-4-Fluorophenyl), 129.8 (d, C2,6-4-Fluorophenyl), 131.1 (C3a,7a-Phthalimide), 132.0 (C1-4-Fluorobenzoyl), 134.3 (C5,6-Phthalimide), 162.8 (d, C4-4-Fluorophenyl), 167.9 (C=O, Phthalimide), 168.2 (C=O, 4-Fluorobenzoyl). IR (KBr, cm-1) ῡ: 3062 (C-H, Stretch, Aromatic), 2924 (C-H, Stretch, Asymmetric, Aliphatic), 2870 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1635 (C=O, Stretch, Amide), 1470, 1284 (C-N, Stretch). MS (m/z, %): 381 (M+, 3), 242 (8), 221 (100), 174 (8), 166 (10), 123 (70), 99 (10), 95 (15), 75 (5), 56 (5).
2-(2-(4-(2-Methoxybenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4g)
1H NMR (CDCl3, 400 MHz) δ (ppm): 1.44 (brs, 4H, Piperazine), 3.14 (brs, 4H, Piperazine), 3.49-4.21 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 6.81-7.49 (m, 4H, 2-Methoxyphenyl), 7.76 (q, 2H, H5,6-Phthalimide), 7.87 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 20.7 (Phthalimide-C-C-Piperazine), 30.3 (C3-Piperazine), 43.9 (C5-Piperazine), 54.4 (C2,6-Piperazine), 56.5 (Phthalimide-C-C-Piperazine), 58.3 (-OCH3), 105 (C6-2-Methoxyphenyl), 113.3 (C4-2-Methoxyphenyl), 120.2 (C4,7-Phthalimide), 124.4 (C2-2-Methoxyphenyl), 131.4 (C5-2-Methoxyphenyl), 132.6 (C3a,7a-Phthalimide), 135.9 (C5,6-Phthalimide), 137.6 (C1-2-Methoxyphenyl), 160.2 (C3-2-Methoxyphenyl), 169.9 (C=O, Phthalimide), 171.1 (C=O, 3-Methoxybenzoyl). MS (m/z, %): 393 (10), 242 (5), 233 (100), 217 (15), 174 (3), 160 (3), 135 (75), 77 (10).
2-(2-(4-(3-Methoxybenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4h)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.01(brs, 4H, Piperazine), 2.98 (brs, 4H, Piperazine), 3.37-4.24 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 7.03 (m, 2H, 3-Methoxyphenyl), 7.36 (m, 1H, 3-Methoxyphenyl), 7.64 (m, 1H, 3-Methoxyphenyl), 7.76 (q, 2H, H5,6-Phthalimide), 7.88 (q, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 36.1 (Phthalimide-C-C-Piperazine), 43.2 (C3-Piperazine), 45.6 (C5-Piperazine), 53.4 (Phthalimide-C-C-Piperazine), 54.8 (C2,6-Piperazine), 56.6 (-OCH3), 118.1 (C6-3-Methoxyphenyl), 120.4 (C4-3-Methoxyphenyl), 123.7 (C4,7-Phthalimide), 124.7 (C2-3-Methoxyphenyl), 125.3 (C5-3-Methoxy-phenyl), 131.6 (C3a,4a-Phthalimide), 134.8 (C5,6-Phthalimide), 135.0 (C1-3-Methoxyphenyl), 159.7 (C3-3-Methoxyphenyl), 166.5 (C=O, Phthalimide), 169.3 (C=O, 3-Methoxybenzoyl). IR (KBr, cm-1) ῡ: 3070 (C-H, Stretch, Aromatic), 2924 (C-H, Stretch, Asymmetric, Aliphatic), 2825 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1639 (C=O, Stretch, Amide), 1462, 1242 (C-N, Stretch). MS (m/z, %): 393 (M+, 3), 242 (12), 233 (100), 217 (12), 174 (10), 135 (70), 99 (15), 77 (15).
2-(2-(4-(4-Methoxybenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4i)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.81 (brs, 4H, Piperazine), 3.00 (brs, 4H, Piperazine), 3.46-4.18 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 7.03 (m, 2H, 3-Methoxyphenyl), 7.41-7.67 (m, 4H, 4-Methoxyphenyl), 7.76 (m, 2H, H5,6-Phthalimide), 7.88 (m, 2H, H4,7-Phthalimide). 13C NMR (DMSO-d6, 62.5 MHz) δ: 35.8 (Phthalimide-C-C-Piperazine), 44.3 (C3-Piperazine), 47.4
(C5-Piperazine), 53.1 (Phthalimide-C-C-Piperazine), 54.5 (C2,6-Piperazine), 57.1 (-OCH3), 114.2 (C3,5-4-Methoxyphenyl), 123.6 (C4,7-4-Phthalimide), 127.8 (C2,6-4-Methoxyphenyl), 128.2 (C1-4-Methoxyphenyl), 132.1 (C3a,7a-Phthalimide), 135.9 (C5,6-4-Phthalimide), 156.9 (C4-4-Methoxyphenyl), 167.1 (C=O, Phthalimide), 169.1 (C=O, 4-Methoxybenzoyl). IR (KBr, cm-1) ῡ: 3080 (C-H, Stretch, Aromatic), 2924 (C-H, Stretch, Asymmetric, Aliphatic), 2858 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1647, 1435, 1253 (C-N, Stretch).
2-(2-(4-(2-Nitrobenzoyl) piperazin-1-yl) ethyl) isoindoline-1,3-dione (4j)
1H NMR (CDCl3, 400 MHz) δ (ppm): 2.96 (brs, 4H, Piperazine), 3.44 (brs, 4H, Piperazine), 3.78-3.94 (m, 4H, Phthalimide-CH2-CH2-Piperazine), 6.93 (m, 2H, 2-Nitrophenyl), 7.43 (m, 1H, 2-Nitrophenyl), 7.75 (m, 2H, H5,6-Phthalimide), 7.86 (m, 2H, H4,7-Phthalimide), 8.01 (m, 1H, 2-Nitrophenyl). 13C NMR (DMSO-d6, 62.5 MHz) δ: 13C NMR (DMSO-d6, 62.5 MHz) δ: 37.4 (Phthalimide-C-C-Piperazine), 43.2 (C3-Piperazine), 45.5 (C5-Piperazine), 53.6 (Phthalimide-C-C-Piperazine), 54.8 (C2,6-Piperazine), 123.7 (C4,7-Phthalimide), 128.6 (C3-2-Nitrophenyl), 128.9 (C1-2-Nitrophenyl), 131.1 (C4-2-Nitorphenyl), 131.7 (C3a,7a-Phthalimide), 132.2 (C6-2-Nitorphenyl), 133.1 (C5-2-Nitorphenyl), 135.4 (C5,6-Phthalimide), 140.6 (C2-2-Nitrophenyl), 166.1 (C=O, Phthalimide), 167.2 (C=O, 2-Nitrobenzoyl). IR (KBr, cm-1) ῡ: 2967 (C-H, Stretch, Asymmetric, Aliphatic), 2927 (C-H, Stretch, Symmetric, Aliphatic), 1712 (C=O, Stretch, Phthalimide), 1650 (C=O, Stretch, Amide), 1608 (C=C, Stretch, Aromatic), 1470 (C=C, Stretch, Aromatic), 1253 (C-N, Stretch).
Docking study
All ligands were docked into the active site of acetylcholinesterase and the binding state and interacted amino acids were compared to donepezil (Figure 4, Figure 5).
Figure 4.
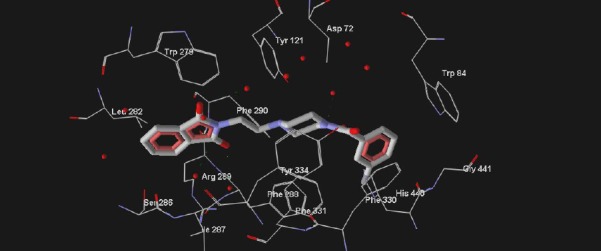
Structure of compound 4e (3-fluoro derivative) in the active site of acetylcholinesterase. The ligand is rendered as stick and the amino acids of the active site of AChE is presented as wireframe. Water molecules provided as red core
Figure 5.
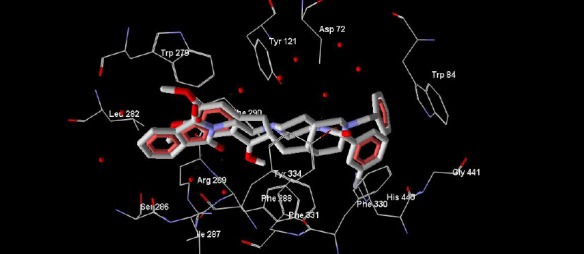
Superimposition of compound 4e with structure of donepezil in the active site of AChE
Enzymatic assay
All prepared compounds (4a-4j) were tested against acetylcholinesterase by Ellman’s test and obtained results were listed in Table 2. Observed potencies were compared with donepezil as reference drug.
Table 2.
Results of anti-acetylcholinesterase activity of compounds 4a-4j (IC50, µM)
| Compound | 4a | 4b | 4c | 4d | 4e | 4f | 4g | 4h | 4i | 4j | Donepezil |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | 2-Cl | 3-Cl | 4-Cl | 2-F | 3-F | 4-F | 2-OCH3 | 3-OCH3 | 4-OCH3 | 2-NO2 | - |
| IC50 (µM) | 333.4 | 70.2 | > 1000 | 3.6 | 7.1* | 50.6 | 100.1 | 1.3 | 20.3* | 140.6 | 0.41 |
IC50 of compounds 4e and 4i have been presented in nanomolar (nM) range
Discussion
Chemistry
A new series of isoindoline-1,3-dione (phthalimide) derivatives were synthesized through the treatment of phthalic anhydride and N-amnioethylpiperazine in the presence of triethylamine as proton acceptor. Refluxing condition was applied in toluene solvent to achieve compound 3 with a moderate yield (61%). Then, compound 3 was used for preparation of final product 4a-4j. Final products were synthesized via a carbodiimide coupling process. Namely, appropriate benzoic acid derivative was mixed with N-ethyl-N-dimethylaminopropyl carbodiimide (EDC) and hydroxybenzotriazole (HOBt) in acetonitrile (CH3CN) solvent and after 30 min equimolar quantities of compound 3 was added. The reaction mixture was stirred for 24 hr. Spectroscopic methods such as 1H NMR, IR and Mass were utilized for characterization of intermediate compound 3 as well as final compounds 4a-4j. Melting point analyser was used to measure the corresponding melting points using open capillary tube and recorded as centigrade degree. Compound 4d with ortho fluorine substituent demonstrated the highest melting point (247 °C), whereas, compounds 4h and 4i with methoxy moiety rendered the lowest melting points (127 °C).
Docking
According to the Figure 3, it is obvious that donepezil has three distinct interactions with acetylcholinesterase enzyme. In the other words, Trp 279, Phe 330 and Trp 84 are the most critical amino acids in the active site of AChE. The binding mode of the tested compounds was investigated by docking method using ArgusLab software. According to the obtained results, there is a similar binding mode and interactions between the docked ligands and donepezil into the active site of acetylcholinesterase (Figure 4, Figure 5). According to the Figure 4, the critical amino acids (Trp 279, Phe 330 and Trp 84) are visible surrounding the docked ligand and also with paying attention to the Figure 5, a similar conformation and orientation like donepezil towards the pivotal amino acids is observable for this ligand in overlaid state.
Structure activity relationship
All final compounds 4a-4j were tested against acetylcholinesterase enzyme and the obtained results were recorded as IC50 in Table 2. Fortunately, the synthesized derivatives demonstrated a remarkable inhibitory activity towards acetylcholinesterase. Various substituents such as Cl, F, methoxy and nitro were introduced on the phenyl ring to explore the impact of electronic effects of the moiety on the potency of these compounds in inhibition of acetylcholine-esterase activity. In the other words, electron withdrawing as well as electron donating moiety were examined. According to the Table 2, it is obvious that both of the electron withdrawing and electron donating moiety have beneficial effect on the potency of the synthesized derivatives. Compound 4e with meta fluorine moiety was the most active compound in this series (IC50 = 7.1 nM). Generally, electron withdrawing groups like chlorine and fluorine at position meta of the phenyl ring provided a better activity compared to positioning of these moieties at ortho and para. Positioning of the methoxy at para (compound 4i, IC50= 20.3 nM) also rendered a favorable potency but lower than compound 4e. Compounds 4d and 4h were also exhibited an acceptable activity in µM range but lower than donepezil. It means that methoxy as an electron donating moiety at position meta could also enhance the anticholinesterase activity in comparison with ortho position. Ortho positioning of chlorine and nitro moieties did not caused a significant increase in activity. It is probable that steric effect that caused by chlorine and nitro moiety be an interrupting factor for proper interaction of these ligands with receptor at position ortho.
Totally, electron withdrawing atoms enhanced the anticholinesterase activity especially at position 3 of the phenyl ring. Increase in electron withdrawing effects was also beneficial for activity. In the other words, replacement of the chlorine with fluorine atom led to the improvement in activity in all positions of the phenyl ring. Electron donating groups is better to substitute at position 3 and 4 of the phenyl ring.
Conclusion
A new series of phthalimide (isoindoline-1,3-dione) derivatives were synthesized and corresponding anti-acetylcholinestetrase activity were assessed using Ellman protocol. Molecular docking was also carried out for exploration of the probable binding mode of the ligands and also for comparison with donepezil. Totally, electron withdrawing groups (Cl, F) at position 3 and electron donating group (methoxy) at position 4 of the phenyl ring enhanced the activity. Compound 4e (m-Fluoro, IC50 = 7.1 nM) and 4i (p-Methoxy, IC50 = 20.3 nM) were the most active compounds in this series and exerted superior potency than donepezil (410 nM). Molecular modeling by docking method also confirmed the results of enzymatic test. Moreover, a similar binding mode was observed for all ligands in superimposition state with donepezil into the active site of acetylcholinesterase.
Acknowledgment
Authors appreciate from the Research Council of Kermanshah University of Medical Sciences for financial supports. This work was performed in partial fulfillment of the requirement for PharmD of Mrs Nasibeh Abdi.
References
- 1.Sugimoto H, Yamanishi Y, Iimura Y, Kawakami Y. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem. 2000;7:303–339. doi: 10.2174/0929867003375191. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Zhu D, Sheng R, Wu H, Hu Y, Wang F, et al. BZYX, a novel acetylcholinesterase inhibitor, significantly improved chemicals-induced learning and memory impairments on rodents and protected PC12 cells from apoptosis induced by hydrogen peroxide. Eur J Pharmacol. 2009;613:1–9. doi: 10.1016/j.ejphar.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Ucar G, Gokhan N, Yesilada A, Bilgin AA. 1-N-Substituted thiocarbamoyl-3-phenyl-5-thienyl-2-pyrazolines: A novel cholinesterase and selective monoamine oxidase B inhibitors for the treatment of Parkinson’s and Alzheimer’s diseases. Neurosci Lett. 2005;382:327–331. doi: 10.1016/j.neulet.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Liston DR, Nielsen JA, Villalobos A, Chapin D, Jones SB, Hubbard ST, et al. Pharmacology of selective acetylcholinesterase inhibitors: implications for use in Alzheimer’s disease. Eur J Pharmacol. 2004;486:9–17. doi: 10.1016/j.ejphar.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 5.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 6.Vitorović-Todorović MD, Juranić IO, Mandić LM, Drakulic BJ. 4-Aryl-4-oxo-N-phenyl-2-aminylbutyramides as acetyl- and butyrylcholinesterase inhibitors. Preparation, anticholinesterase activity, docking study, and 3D structure-activity relationship based on molecular interaction fields. Bioorg Med Chem. 2010;18:1181–1193. doi: 10.1016/j.bmc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 7.Kapková P, Alptüzün V, Frey P, Erciyasb E, Holzgrabe U. Search for dual function inhibitors for Alzheimer’s disease: Synthesis and biological activity of acetylcholinesterase inhibitors of pyridinium-type and their Aβfibril formation inhibition capacity. Bioorg Med Chem. 2006;14:472–478. doi: 10.1016/j.bmc.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto H, Iimura Y, Yamanishi Y, Yamatsu K. Synthesis and Structure-Activity Relationships of Acetylcholinesterase Inhibitors: 1-Benzyl-4-[(5,6-dimethoxy-l-oxoindan-2yl)methyl]piperidine Hydrochloride and Related Compounds. J Med Chem. 1995;24:4821–4829. doi: 10.1021/jm00024a009. [DOI] [PubMed] [Google Scholar]
- 9.Foroumadi A, Mohammadi-Farani A, Garmsiri Mahvar M, Aliabadi A. Synthesis and evaluation of anti-acetylcholinesterase activity of 2-(2-(4-(2-Oxo-2-phenylethyl)piperazin-1-yl)ethyl)isoindoline-1,3-dione derivatives with potential anti-Alzheimer effects. Iran J Basic Med Sci. 2013;10:1049–1054. [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadi-Farani A, Ahmadi A, Nadri H, Aliabadi A. Synthesis, docking and acetylcholinesterase inhibitory assessment of 2-(2-(4-Benzylpiperazin-1-yl)ethyl)isoindoline-1,3-dione with potential anti-alzheimer effects. Daru. 2013;21:47–55. doi: 10.1186/2008-2231-21-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q, Yang G, Mei X, Yuan H, Ning J. Novel acetylcholinesterase inhibitors: Synthesis and structure-activity relationships of phthalimide alkyloxyphenyl N,N-dimethylcarbamate derivatives. Pestic Biochem Physiol. 2009;95:131–134. [Google Scholar]
- 12.Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept®): implications for the design of new anti-Alzheimer drugs. Structure. 1999;3:297–307. doi: 10.1016/s0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 13.Alonso D, Dorronsoro I, Rubio L, Munoz P, García-Palomero E, Del Monte M, et al. Donepezil-tacrine hybrid related derivatives as new dual binding site inhibitors of AChE. Bioorg Med Chem. 2005;13:6588–6597. doi: 10.1016/j.bmc.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Mary A, Zafiarisoa Renko D, Guillou C, Thal C. Potent acetylcholinesterase inhibitors: design, synthesis, and structure-activity relationships of bis-interacting ligands in the galanthamine series. Bioorg Med Chem. 1998;6:1835–1850. doi: 10.1016/s0968-0896(98)00133-3. [DOI] [PubMed] [Google Scholar]
- 15.Ragavendran JV, Sriram D, Patel SK, Reddy IV, Bharathwajan N, Stables J. Design and synthesis of anticonvulsants from a combined phthalimide-GABA-anilide and hydrazone pharmacophore. Eur J Med Chem. 2007;42:146–151. doi: 10.1016/j.ejmech.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi-Farani A, Foroumadi A, Rezvani Kashani M, Aliabadi A. N-Phenyl-2-p-tolylthiazole-4-carboxamide derivatives: Synthesis and cytotoxicity evaluation as anticancer agents. Iran J Basic Med Sci. 2014;17:502–508. [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadi-Farani A, Heidarian N, Aliabadi A. N-(5-Mercapto-1,3,4-thiadiazol-2-yl)-2-phenylacetamide derivatives: Synthesis and in vitro cytotoxcity evaluation as potential anticancer agents. Iran J Pharm Res. 2014;12:487–492. [PMC free article] [PubMed] [Google Scholar]
- 18.Aliabadi A, Foroumadi A, Safavi M, Kaboudian Ardestani S. Synthesis, molecular docking and cytotoxicity evaluation of 2-(4-substituted-benzyl)isoindoline-1,3-dione derivatives as anticancer agents. J Rep Pharm Sci. 2012;1:19–22. [Google Scholar]
- 19.Aliabadi A, Shamsa F, Ostad SN, Emami S, Shafiee A, Davoodi J, et al. Synthesis and biological evaluation of 2-Phenylthiazole-4-carboxamide derivatives as anticancer agents. Eur J Med Chem. 2010;11:5384–5389. doi: 10.1016/j.ejmech.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 20.ArgusLab 4.0 Mark A. Seattle, WA: Thompson Planaria Software LLC; http://www.arguslab.com/arguslab.com/ArgusLab.html . [Google Scholar]
- 21. http://RCSB.org .
- 22.Fernandez D, Aviles FX, Vendrell J. A new type of five-membered heterocyclic inhibitors of basic metallocarboxypeptidases. Eur J Med Chem. 2009;44:3266–3271. doi: 10.1016/j.ejmech.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 23. http://www.molegro.com .
- 24.Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 25.Nadri H, Pirali-Hamedani M, Shekarchi M, Abdollahi M, Sheibani V, Amanlou M, et al. Design, synthesis and anticholinesterase activity of a novel series of 1-benzyl-4-((6-alkoxy-3-oxobenzofuran-2(3H)-ylidin)methyl) pyridinium derivatives. Bioorg Med Chem. 2010;18:6360–6366. doi: 10.1016/j.bmc.2010.07.012. [DOI] [PubMed] [Google Scholar]


