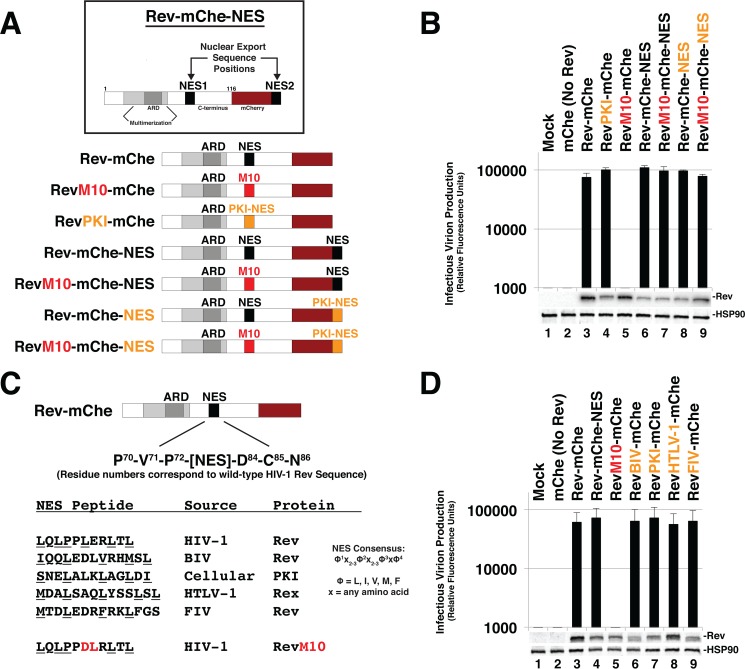
FIG 1.
Rev is robustly tolerant of changes to NES number, position, or identity. (A) Cartoon indicating Rev-mCherry (Rev-mChe) variants and relevant NES positions or modifications, native position (NES1), altered identity (PKI), inactivated (M10), and C-terminal position (NES2). Rev's arginine-rich domain (ARD, amino acids 34 to 50) encodes the nuclear localization signal (NLS) and RNA-binding activities. Rev's native NES (NES1 position; amino acids 73 to 83) is located within the disordered C-terminal domain (B) Capacity of Rev variants depicted in 1A to trans-complement Rev-minus HIV-1 YFP reporter viruses. 293T cells were transfected with plasmids encoding full-length, NL4-3-derived E-R-Rev-/YFP reporter proviruses, VSV-G, and either mCherry alone (No Rev control) or the indicated Rev-mChe variant. The lane 1 control lacks proviral DNA. Cell lysates and supernatants were harvested at 48 h posttransfection and processed for immunoblotting, and equivalent amounts of supernatant were used to infect target HeLa cells in order to gauge infectious virion production based on YFP fluorescence at 48 h postinfection (viral infectivity assay). Error bars represent standard deviations from the mean for three independent experiments. Rev and HSP90 (loading control) were detected using anti-Rev and anti-HSP90 antisera. (C) Depiction of additional Rev-mChe variants bearing alternative NES sequences. Predicted NES consensus-defining amino acids are underlined. BIV, bovine immunodeficiency virus; PKI, protein kinase A inhibitor; HTLV-1, human T-lymphotropic virus type 1; FIV, feline immunodeficiency virus. (D) Activities of the Rev variants shown in panel C were determined by viral infectivity assay as described for panel B.