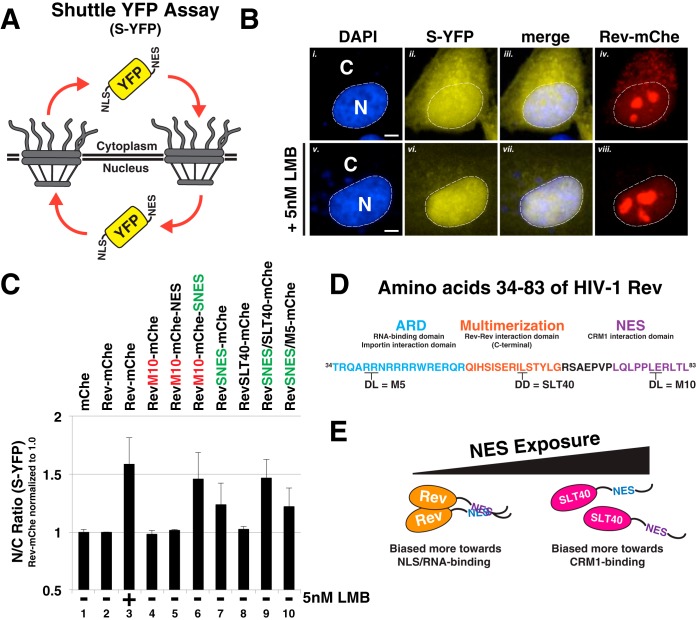
FIG 6.
Rev's capacity to mask a SNES is both context and multimerization dependent. (A) Diagram depicting shuttle YFP (S-YFP) reporter assay. This assay employs NLS- and NES-directed nucleocytoplasmic trafficking of S-YFP reporter to determine the disruptive effect of Rev variants encoding different NES configurations. (B) Inhibition of CRM1-dependent export promotes S-YFP nuclear accumulation. The image panel depicts individual transfected HeLa cells producing S-YFP and the indicated Rev variant (the example shown is Rev-mChe) in the absence (panels i to iv) or presence (panels v to viii) of 5 nM leptomycin B (LMB) as a control for CRM1-dependent nuclear export inhibition. Transfected HeLa cells were fixed at 24 h posttransfection and DAPI stained to demarcate nuclear and cytoplasmic compartments (nuclear border labeled by white dotted line). Scale bars, 10 μm. (C) Multimerization-deficient Rev encoding SNES in native context phenocopies C-terminal SNES and LMB treatment. The nuclear-to-cytoplasmic ratio (N/C ratio) of YFP fluorescence was measured from individual cells producing the indicated Rev variants in an S-YFP assay. Cells were transfected to express the indicated Rev variant and viral RNA and processed as for panel B. Nuclear and cytoplasmic YFP fluorescence was measured from YFP- and mCherry-positive HeLa cells and assigned using DAPI as a marker for nuclear-specific fluorescence signal for reference (see Fig. 6B, compare panels i to ii to panels v to vi). Error bars represent the standard deviations from the mean for four independent experiments (>250 cells measured per condition). Automated cell segmentation and fluorescence quantification were performed using KNIME/FIJI. (D) Wild-type HIV-1 Rev sequence (amino acids 34 to 83) indicating functional activities and amino acid substitutions conferring loss of RNA binding (M5), Rev multimerization (SLT40), and CRM1 binding (M10). (E) Model for regulation of multimerization-dependent NES masking. Physical masking of SNES in native NES1 context decreases interaction potential with CRM1, thus promoting the cytoplasmic accumulation of S-YFP, as measured in panel C.