ABSTRACT
H7 subtype influenza A viruses are widely distributed and have been responsible for human infections and numerous outbreaks in poultry with significant impact. Despite this, the disease-causing potential of the precursor low-pathogenic (LP) H7 viruses from the wild bird reservoir has not been investigated. Our objective was to assess the disease-causing potential of 30 LP H7 viruses isolated from wild avian species in the United States and Canada using the DBA/2J mouse model. Without prior mammalian adaptation, the majority of viruses, 27 (90%), caused mortality in mice. Of these, 17 (56.7%) caused 100% mortality and 24 were of pathogenicity similar to that of A/Anhui/1/2013 (H7N9), which is highly pathogenic in mice. Viruses of duck origin were more pathogenic than those of shorebird origin, as 13 of 18 (72.2%) duck origin viruses caused 100% mortality while 4 of 12 (33.3%) shorebird origin viruses caused 100% mortality, despite there being no difference in mean lung viral titers between the groups. Replication beyond the respiratory tract was also evident, particularly in the heart and brain. Of the 16 viruses studied for fecal shedding, 11 were detected in fecal samples. These viruses exhibited a strong preference for avian-type α2,3-linked sialic acids; however, binding to mammalian-type α2,6-linked sialic acids was also detected. These findings indicate that LP avian H7 influenza A viruses are able to infect and cause disease in mammals without prior adaptation and therefore pose a potential public health risk.
IMPORTANCE Low-pathogenic (LP) avian H7 influenza A viruses are widely distributed in the avian reservoir and are the precursors of numerous outbreaks of highly pathogenic avian influenza viruses in commercial poultry farms. However, unlike highly pathogenic H7 viruses, the disease-causing potential of LP H7 viruses from the wild bird reservoir has not been investigated. To address this, we studied 30 LP avian H7 viruses isolated from wild avian species in the United States and Canada using the DBA/2J mouse model. Surprisingly, the majority of these viruses, 90%, caused mortality in mice without prior mammalian adaptation, and 56.7% caused 100% mortality. There was also evidence of spread beyond the respiratory tract and fecal shedding. Therefore, the disease-causing potential of LP avian H7 influenza A viruses in mammals may be underestimated, and these viruses therefore pose a potential public health risk.
KEYWORDS: H7, influenza, avian viruses, viral pathogenesis
INTRODUCTION
Low-pathogenic (LP) avian influenza A viruses (AIVs) of the H5 and H7 subtypes in wild avian species are unique compared to the other 14 AIV subtypes, as they can become highly pathogenic and infect both poultry and mammalian species, including humans, seals, swine, and horses (1–5). The first studied AIV was an H7 virus originally described by Perroncito in northern Italy in 1878 as “fowl plague” (6). Our long-term surveillance of North American shorebirds, including those at Delaware Bay and in ducks in Alberta, Canada, has yielded numerous H7 LP AIVs. Our previous phylogenetic study of these viruses supported the hypothesis that highly pathogenic avian influenza (HPAI) viruses emerged from the pool of LP AIVs in the wild bird reservoir (7). However, the potential for these H7 LP AIVs to cause disease in mammals has not been studied. This is the focus of the present study.
Diseases in poultry infected with H7 AIVs can be severe and are often lethal, as evidenced by an outbreak in the Netherlands in 2003, where a HPAI H7N7 virus resulted in the destruction of more than 30 million birds and in 89 confirmed human infections (8–10). There was also evidence of limited human-to-human spread during the outbreak (10). The outbreak was not unique, as there have been numerous outbreaks of H7N1 and H7N3 viruses in poultry in several countries, including Italy, Mexico, Canada, and Pakistan, resulting in the destruction of millions of birds and significant economic impact (reviewed in reference 11). Recently, the presence of HPAI H7N8 viruses in a commercial turkey flock in Indiana was reported by the U.S. Department of Agriculture (12).
Most human H7 influenza A virus infections have been associated with poultry contact (1). Therefore, in the event of a large-scale outbreak in poultry, increased human contact may be an important risk factor. The propensity for H7 viruses to infect humans may also be linked to partial recognition of α2,6-linked sialic acids (13, 14) and ocular tropism (15), as conjunctivitis has occurred in more than 80% of human H7 infections (10, 16). Based on results from BALB/c mice challenged with isogenic chimeric AIVs, the H7 subtype demonstrated a potential to cause enhanced disease in mammals (17). H7N7 and H7N9 viruses of human origin can also induce a transcriptomic response resulting in increased pathogenicity in BALB/c mice (18).
An H7N9 virus was responsible for a recent influenza outbreak in China beginning in 2013 (19). The virus was of avian origin and was transmitted to humans via infected poultry. Although rare, human-to-human transmission events have also been documented (20). Further, two viruses isolated during the initial outbreak showed efficient direct contact and respiratory droplet contact in the ferret model, indicating potential for airborne human-to-human transmission (21, 22). Since the initial outbreak, there have been two subsequent peaks of human infections, and H7N9 viruses have become established in chickens and have spread to new geographic areas, raising concerns for further outbreaks and reassortment events giving rise to new viruses (23).
In general, AIVs of the H7 subtype have caused the greatest number of human infections compared to other AIV subtypes, and it appears that a much broader variety of H7 viruses than of other AIV subtypes can cause human infections (reviewed in reference 11). This is evident from the fact that human infections with H7 AIVs of both low and high pathogenicity from both Eurasian and North American lineages have been documented, while only Eurasian lineage H5, H6, H9, and H10 AIVs have been documented to cause human cases (reviewed in reference 11). There is also evidence that H7 viruses are less immunogenic than other subtypes, which, from a vaccination perspective, is of particular concern (24–27). Because H7 viruses appear to stand out from other AIV subtypes in the variety of viruses that can cause disease, it is important to study these viruses in mammalian models to further understand their potential to cause disease. As previous studies have focused on HPAI viruses of the H5 and H7 subtypes, not LP subtype H7 viruses, knowledge of these viruses is limited. As such, the objective of this study was to assess the potential of historic and contemporary North American LP H7 AIVs to cause disease in mammals using the mouse model.
RESULTS
Avian H7 viruses cause lethal infections in DBA/2J mice.
We selected 30 H7Nx AIVs, 12 of Charadriiformes (shorebird) origin and 18 of Anseriformes (duck) origin. Shorebird origin viruses were collected during our surveillance activities at Delaware Bay, and the majority of the duck origin viruses were obtained during surveillance activities in Alberta, Canada. Other duck viruses were obtained in Texas, Minnesota, and Louisiana. We inoculated DBA/2J mice to determine the capacity of H7 AIVs to cause disease in mammals. On the basis of mortality and morbidity, viruses were assigned pathogenicity indices (PIs), with 4 being the most pathogenic and 1 being the least pathogenic. PI values were calculated using percent survival and percent weight loss by time. PI-4 and PI-3 viruses caused 100% mortality and PI-2 viruses caused between 80% and 20% mortality, while PI-1 viruses were not lethal (Table 1).
TABLE 1.
Mortality rates and pathogenicity in DBA/2J mice infected with avian viruses at 106 EID50
| Virus name | Subtype | Mortality (%) | Survival scorea | Weight loss scoreb | Total pathogenicity scorec | PId |
|---|---|---|---|---|---|---|
| A/blue-winged teal/TX/578588/2002 | H7N1 | 100 | 0.240 | 0.060 | 0.300 | 4 |
| A/ruddy turnstone/NJ/65/1985 | H7N3 | 100 | 0.251 | 0.049 | 0.301 | 4 |
| A/pintail/Alberta/25/2001 | H7N3 | 100 | 0.257 | 0.06 | 0.318 | 4 |
| A/mallard/Alberta/195/1989 | H7N3 | 100 | 0.274 | 0.072 | 0.347 | 4 |
| A/blue-winged teal/TX/AI12-909/2012 | H7N1 | 100 | 0.286 | 0.072 | 0.358 | 4 |
| A/laughing gull/Delaware Bay/42/2006 | H7N3 | 100 | 0.309 | 0.072 | 0.380 | 4 |
| A/shorebird/DE/22/2006 | H7N3 | 100 | 0.297 | 0.084 | 0.381 | 4 |
| A/mallard/Alberta/699/1981 | H7N3 | 100 | 0.300 | 0.082 | 0.382 | 4 |
| A/mallard/Alberta/93/1996 | H7N3 | 100 | 0.229 | 0.071 | 0.399 | 4 |
| A/mallard/Alberta/590/2010 | H7N8 | 100 | 0.331 | 0.075 | 0.406 | 4 |
| A/ruddy turnstone/Delaware Bay/262/2006 | H7N3 | 100 | 0.331 | 0.075 | 0.407 | 4 |
| A/blue-winged teal/MN/AI09-3760/2009 | H7N3 | 100 | 0.377 | 0.087 | 0.464 | 4 |
| A/mallard/Alberta/243/2006 | H7N3 | 100 | 0.389 | 0.096 | 0.485 | 4 |
| A/mallard/Alberta/49/1976 | H7N3 | 100 | 0.400 | 0.108 | 0.508 | 3 |
| A/green-winged teal/Alberta/228/1985 | H7N3 | 100 | 0.446 | 0.092 | 0.538 | 3 |
| A/mallard/MN/AI08-5469/2008 | H7N3 | 100 | 0.457 | 0.103 | 0.561 | 3 |
| A/mallard/MN/AI07-3433/2007 | H7N3 | 100 | 0.469 | 0.116 | 0.584 | 3 |
| A/shorebird/Delaware Bay/2696/1988 | H7N3 | 80 | 0.457 | 0.180 | 0.637 | 2 |
| A/ruddy turnstone/NJ/AI11-2098/2011 | H7N3 | 60 | 0.457 | 0.184 | 0.641 | 2 |
| A/blue-winged teal/LA/AI11-2911/2011 | H7N3 | 80 | 0.491 | 0.174 | 0.665 | 2 |
| A/mallard/Alberta/167/2010 | H7N3 | 80 | 0.503 | 0.191 | 0.694 | 2 |
| A/mallard/Alberta/192/2010 | H7N3 | 80 | 0.549 | 0.180 | 0.728 | 2 |
| A/ruddy turnstone/NJ/AI11-1678/2011 | H7N7 | 80 | 0.557 | 0.192 | 0.749 | 2 |
| A/ruddy turnstone/Delaware Bay/126/1996 | H7N3 | 60 | 0.583 | 0.180 | 0.763 | 2 |
| A/mallard/MN/AI09-3770/2009 | H7N9 | 80 | 0.594 | 0.183 | 0.778 | 2 |
| A/laughing gull/DE/AI00-2455/2000 | H7N3 | 40 | 0.617 | 0.192 | 0.809 | 2 |
| A/mallard/MN/19033/1999 | H7N7 | 20 | 0.731 | 0.184 | 0.915 | 1 |
| A/red knot/Delaware Bay/242/1994 | H7N7 | 0 | 0.800 | 0.190 | 0.990 | 1 |
| A/ruddy turnstone/DE/AI00-1538/2000 | H7N9 | 0 | 0.800 | 0.194 | 0.994 | 1 |
| A/shorebird/Delaware Bay/285/1994 | H7N7 | 0 | 0.800 | 0.196 | 0.996 | 1 |
The survival score was calculated as follows: 0.8 × (survival AUC/maximum AUC).
The weight loss score was calculated as follows: 0.2 × (weight loss AUC/maximum AUC).
The total pathogenicity score was the sum of the survival and weight loss scores.
Pathogenicity indices classify viruses as follows: most pathogenic (4) to least pathogenic (1).
Of the 30 isolates, 13 (43.3%) were PI-4, 4 (13.3%) were PI-3, 9 (30%) were PI-2, and 4 (13.3%) were PI-1. Of these, 27 (90%) caused mortality in mice and 17 (56.7%) caused 100% mortality. Only three viruses did not cause mortality: A/red knot/Delaware Bay/242/1994 (H7N7) (PI-1), A/ruddy turnstone/DE/AI00-1538/2000 (H7N9) (PI-1), and A/shorebird/Delaware Bay/285/1994 (H7N7) (PI-1) (Table 1).
Duck origin viruses appeared more pathogenic than those from shorebirds, as 13 of 18 duck origin viruses (72.2%) caused 100% mortality compared to 4 of 12 shorebird origin viruses that caused 100% mortality. This was also evident from the PI classifications, as 13 of 18 (72.2%) duck origin viruses were either PI-4 or PI-3, while 4 of 12 (33.3%) viruses of shorebird origin were PI-4 and none were PI-3 (Fig. 1). Duck origin viruses also significantly reduced the survival rate in mice compared to shorebird origin viruses. Survival rates were similar to that produced by A/Anhui/1/2013 (H7N9), which is highly pathogenic in mice (Fig. 2A). Furthermore, duck isolates caused significantly greater weight loss in mice than shorebird isolates at 6, 7, 8, and 9 days postinoculation (dpi) (P < 0.05, 0.01, 0.001, and 0.01, respectively) (Fig. 2B).
FIG 1.
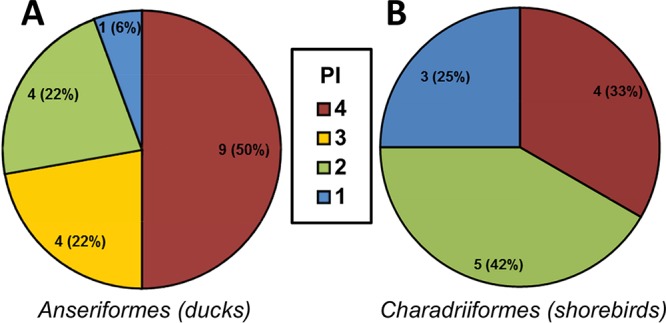
Classification of H7 viruses by PI revealed that duck origin viruses were more pathogenic in mice than shorebird origin viruses. The pie charts show the number of duck origin (A) and shorebird origin (B) H7 viruses in each PI. PI-4 viruses were the most pathogenic on the basis of mortality and morbidity, while PI-1 viruses were the least pathogenic by these measures. Of the 18 duck origin viruses, 9 were classified as PI-4, 4 as PI-3, 4 as PI-2, and 1 as PI-1. Of the 12 shorebird origin viruses, 4 were classified as PI-4, none were PI-3, 5 were PI-2, and 3 were PI-1. The classifications are based on data presented in Table 1.
FIG 2.
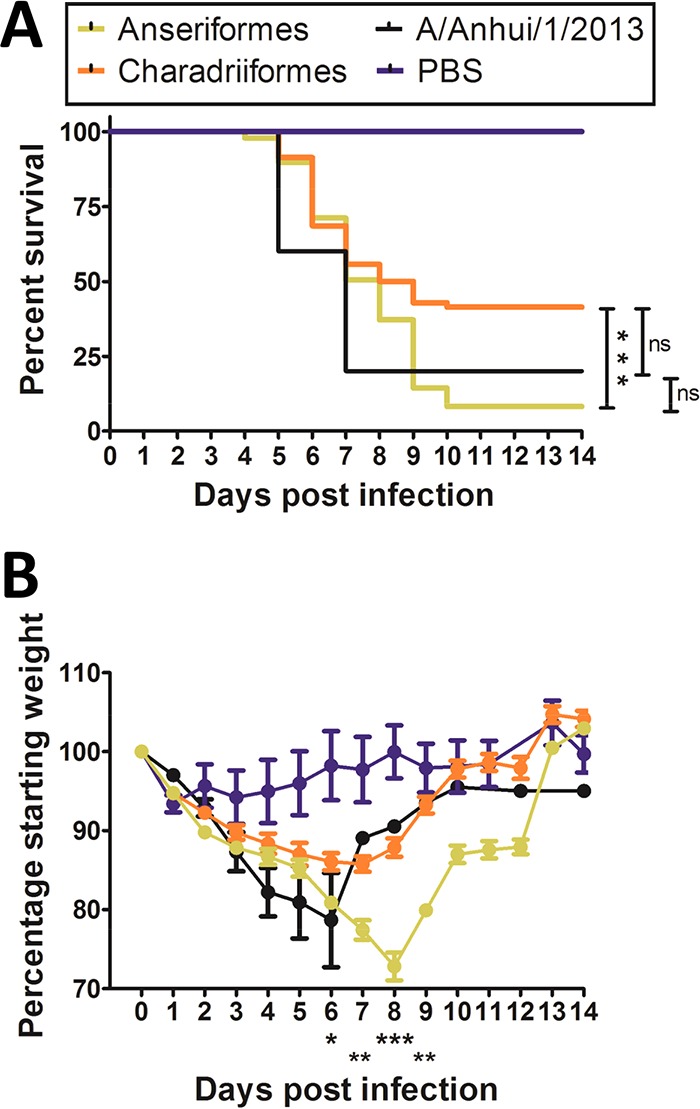
Duck (Anseriformes) origin H7 viruses cause greater mean mortality and weight loss in mice than shorebird (Charadriiformes) origin viruses. (A) The mean survival of mice inoculated with duck origin viruses was significantly less than that of mice inoculated with shorebird origin viruses and similar to that of mice inoculated with A/Anhui/1/2013 (H7N9). (B) Mean weight loss in mice inoculated with duck origin viruses was significantly greater on days 6 to 9 postinfection than that of mice inoculated with shorebird origin viruses and was similar to the mean weight loss in mice inoculated with A/Anhui/1/2013 (H7N9). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant. Error bars indicate standard errors of the means (SEM).
Based on the neuraminidase (NA) subtypes, viruses fell into two broad categories: those with pathogenicity similar to and those with pathogenicity lower than that of A/Anhui/1/2013 (H7N9), which caused 80% mortality (Fig. 3A). Two virus subtypes caused significantly less mortality than A/Anhui/1/2013 (H7N9): H7N7 (n = 4 viruses) and H7N9 (n = 2 viruses) (P < 0.01 and P < 0.05, respectively) (Fig. 3A). The remaining subtypes, H7N1 (n = 2), H7N3 (n = 21), and H7N8 (n = 1), were of pathogenicity similar to that of A/Anhui/1/2013 (H7N9). This was also reflected by the weight loss caused by these viruses, as the mice inoculated with H7N7 or H7N9 viruses lost significantly less weight at 3 to 5 dpi than mice inoculated with A/Anhui/1/2013 (H7N9) (Fig. 3B). It should be noted that the analysis was limited by the vast majority of these viruses being H7N3 subtypes; however, H7N3 tends to be more prevalent in North American ducks and shorebirds than other H7 subtypes. It should also be noted that the analysis was limited by the fact that, with the exception of H7N3, the NA subtypes were not well represented.
FIG 3.
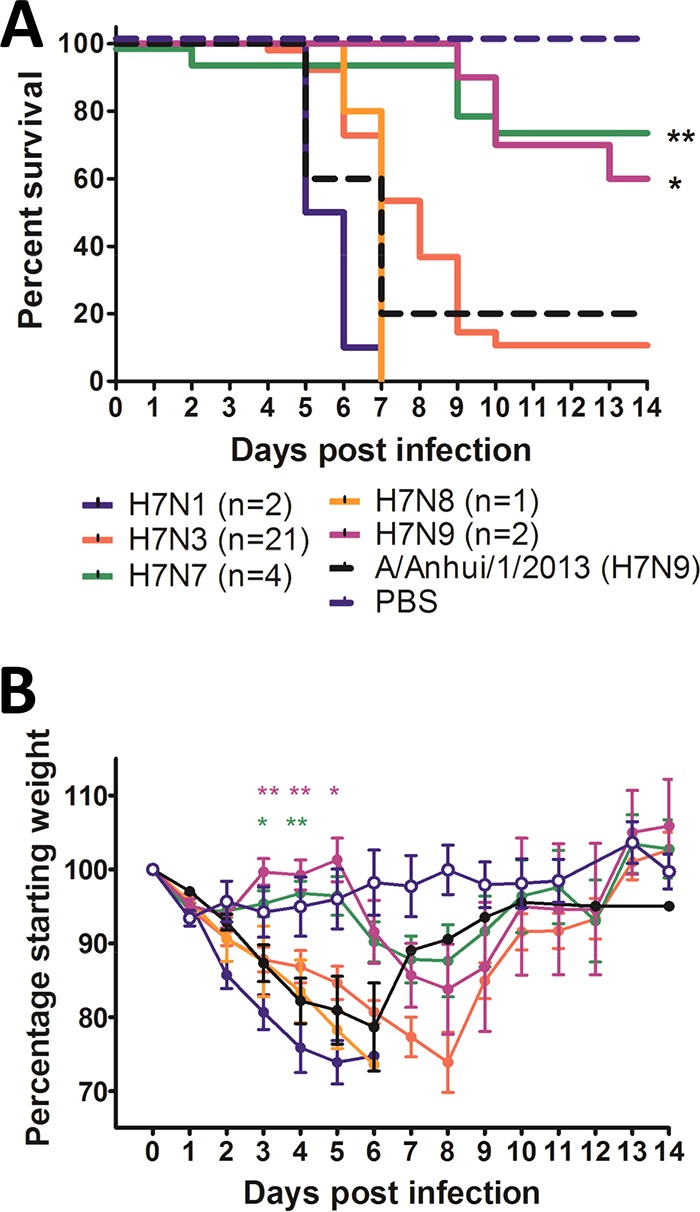
The pathogenicity of H7 influenza viruses was not restricted by NA subtype. (A) Classification by NA subtype revealed that H7N7 and H7N9 viruses were significantly less pathogenic than A/Anhui/1/2013 (H7N9). (B) Classification by NA subtype revealed that H7N7 and H7N9 viruses caused significantly less weight loss in mice on days 3 to 5 than A/Anhui/1/2013 (H7N9). The weight loss caused by viruses of other NA subtypes was similar to that of A/Anhui/1/2013 (H7N9). *, P < 0.05; **, P < 0.01. Error bars indicate SEM.
H7 viruses can replicate in the lung and in extrapulmonary tissues.
To study the replication of H7 viruses in various tissues, organs were collected from mice when they reached humane endpoints and were euthanized, and virus titers were measured by 50% egg infectious dose (EID50). We detected virus in the lungs of all inoculated mice, with the exception of A/ruddy turnstone/DE/AI00-1538/2000 (H7N9) and A/mallard/MN/19033/1999 (H7N7) (Table 2). Viruses of both duck and shorebird origin replicated to similar mean titers in the lung (duck origin, 5.91 ± 0.11 EID50/ml; shorebird origin, 6.11 ± 0.18 EID50/ml) (Fig. 4A). Almost all of the viruses that caused mortality were detected in the brain, albeit in a minority of animals (Fig. 4B and Table 2). No significant difference in the mean brain viral titers was observed between the groups. Virus was detected in the intestine for 6 of the 30 viruses, and these viruses caused 80 to 100% mortality (Table 2). Unlike the brain, detection of virus in the intestine appeared more robust, as 60 to 100% positivity rates were observed (Table 2). As viruses were detected in the gastrointestinal tracts of some mice, we studied fecal samples obtained from mice inoculated with a selection of viruses from each PI to determine if the viruses were shed in feces. Fecal shedding was not restricted to the most pathogenic viruses and was observed from mice inoculated with viruses of all PIs (Table 3).
TABLE 2.
Mortality rates and mean viral titers in the lung associated with North American H7 IAV infection
| Virus name | Subtype | Mortality (%) | Mean lung titera | Virus detectionb in: |
|
|---|---|---|---|---|---|
| Brain | Intestine | ||||
| A/blue-winged teal/TX/578588/2002 | H7N1 | 100 | 5.45 ± 0.0.17 | 2/5 | 3/5 |
| A/ruddy turnstone/NJ/65/1985 | H7N3 | 100 | 7.00 ± 0.25 | 1/5 | − |
| A/pintail/Alberta/25/2001 | H7N3 | 100 | 5.38 ± 0.63 | 1/4 | − |
| A/mallard/Alberta/195/1989 | H7N3 | 100 | 4.25 | 1/5 | − |
| A/blue-winged teal/TX/AI12-909/2012 | H7N1 | 100 | 6.66 ± 0.48 | 2/5 | |
| A/laughing gull/Delaware Bay/42/2006 | H7N3 | 100 | 6.38 ± 0.88 | 1/5 | − |
| A/shorebird/DE/22/2006 | H7N3 | 100 | 5.27 ± 0.58 | 1/5 | − |
| A/mallard/Alberta/699/1981 | H7N3 | 100 | 5.88 ± 0.38 | 1/4 | − |
| A/mallard/Alberta/93/1996 | H7N3 | 100 | 3.88±.38 | − | − |
| A/mallard/Alberta/590/2010 | H7N8 | 100 | 6.42 ± 0.22 | 2/5 | − |
| A/ruddy turnstone/Delaware Bay/262/2006 | H7N3 | 100 | 6.88 ± 0.63 | 1/5 | − |
| A/blue-winged teal/MN/AI09-3760/2009 | H7N3 | 100 | 6.67 ± 0.24 | 1/5 | − |
| A/mallard/Alberta/243/2006 | H7N3 | 100 | 4.50 ± 0.25 | 1/5 | − |
| A/mallard/Alberta/49/1976 | H7N3 | 100 | 5.58 ± 0.29 | 3/5 | − |
| A/green-winged teaL/Alberta/228/1985 | H7N3 | 100 | 4.88 ± 0.41 | − | 5/5 |
| A/mallard/MN/AI08-5469/2008 | H7N3 | 100 | 6.87 ± 0.56 | 2/5 | 4/5 |
| A/mallard/MN/AI07-3433/2007 | H7N3 | 100 | 6.04 ± 0.05 | 2/5 | − |
| A/shorebird/Delaware Bay/2696/1988 | H7N3 | 80 | 6.37 ± 0.13 | − | − |
| A/ruddy turnstonE/NJ/AI11-2098/2011 | H7N3 | 60 | 4.21 ± 0.18 | − | − |
| A/blue-winged teal/LA/AI11-2911/2011 | H7N3 | 80 | 6.44 ± 0.28 | 2/4 | 4/4 |
| A/mallard/Alberta/167/2010 | H7N3 | 80 | 5.13 ± 0.38 | 1/4 | − |
| A/mallard/Alberta/192/2010 | H7N3 | 80 | 5.99 ± 0.08 | 1/4 | 4/4 |
| A/ruddy turnstone/NJ/AI11-1678/2011 | H7N7 | 80 | 5.84 ± 0.77 | − | − |
| A/ruddy turnstone/Delaware Bay/126/1996 | H7N3 | 60 | 4.88 ± 0.88 | 1/3 | − |
| A/mallard/MN/AI09-3770/2009 | H7N9 | 80 | 5.04 ± 0.92 | − | 3/4 |
| A/laughing gull/DE/AI00-2455/2000 | H7N3 | 40 | 5.70 ± 0.17 | 1/2 | − |
| A/mallard/MN/19033/1999 | H7N7 | 20 | 0 | − | − |
| A/red knot/Delaware Bay/242/1994 | H7N7 | 0 | 6.75 | ND | − |
| A/ruddy turnstone/DE/AI00-1538/2000 | H7N9 | 0 | 0 | ND | − |
| A/shorebird/Delaware Bay/285/1994 | H7N7 | 0 | 6.58 ± 0.23 | ND | − |
Viral titers are expressed as EID50/ml ± standard error of the mean.
Tissue samples were screened in eggs to determine the number of mice positive per total number screened. ND, not done. −, no animals were positive.
FIG 4.
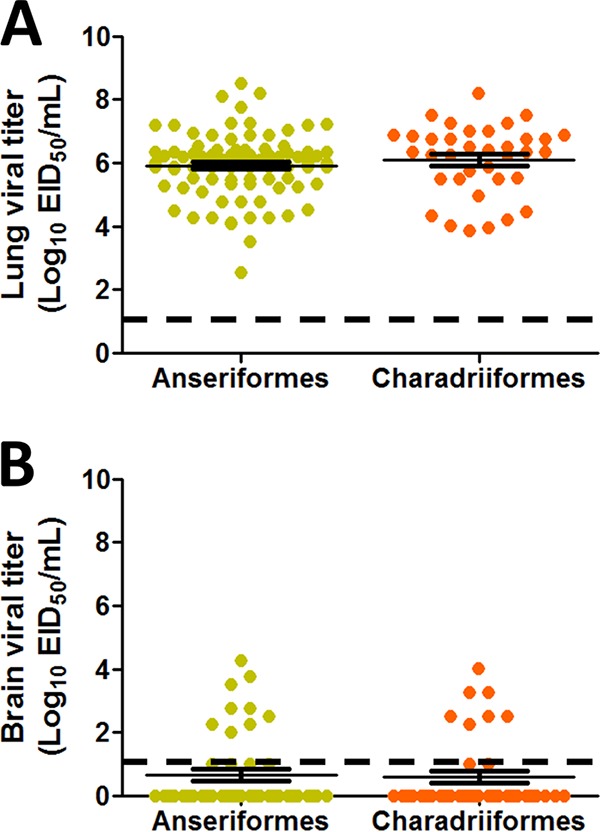
H7 viruses replicated in the lungs and showed limited replication in the brains of infected mice. (A) All viruses replicated in the lungs of inoculated mice. The mean viral lung titers of both duck (Anseriformes) and shorebird (Charadriiformes) origin viruses were similar. (B) Viral replication in the brain was limited to nine mice inoculated with duck origin viruses and seven mice inoculated with shorebird origin viruses, and titers were relatively low compared to lung viral titers. Error bars indicate SEM.
TABLE 3.
Fecal shedding of selected viruses of each PI
| Virus name | Subtype | PI | Fecal virus shedding (EID50/ml), where determineda |
||
|---|---|---|---|---|---|
| 2 dpi | 3 dpi | 6 dpi | |||
| A/mallard/Alberta/699/1981 | H7N3 | 4 | + (1.5) | + | − |
| A/mallard/Alberta/195/1989 | H7N3 | 4 | − | − | − |
| A/mallard/Alberta/93/1996 | H7N3 | 4 | − | − | - |
| A/pintail/Alberta/25/2001 | H7N3 | 4 | + (1.5) | − | − |
| A/mallard/Alberta/243/2006 | H7N3 | 4 | − | − | − |
| A/ruddy turnstone/NJ/65/1985 | H7N3 | 4 | − | − | − |
| A/ruddy turnstone/Delaware Bay/290/2006 | H7N4 | 4 | + (1.25) | − | − |
| A/ruddy turnstone/Delaware Bay/262/2006 | H7N5 | 4 | + (1.5) | − | − |
| A/laughing gull/Delaware Bay/42/2006 | H7N3 | 4 | + (2.25) | + | − |
| A/mallard/Alberta/590/2010 | H7N8 | 4 | − | + (1.25) | − |
| A/shorebird/Delaware Bay/2696/1988 | H7N3 | 2 | + (1.5) | + | − |
| A/ruddy turnstone/NJ/AI11-1678/2011 | H7N7 | 2 | + (1.5) | + | − |
| A/ruddy turnstone/Delaware Bay/126/1996 | H7N3 | 2 | + (1.5) | + | − |
| A/mallard/Alberta/167/2010 | H7N3 | 2 | + (1.25) | + | − |
| A/shorebird/Delaware Bay/285/1994 | H7N7 | 1 | − | − | − |
| A/red knot/Delaware Bay/242/1994 | H7N7 | 1 | − | + (2.21) | − |
+, positive; −, negative. Samples were screened in 3 eggs and classified as positive if all three eggs were positive by hemagglutination. The EID50 (in parentheses) was then determined for these samples.
Time course of extrapulmonary spread of H7 viruses with differing pathogenicity indices.
To further study the extrapulmonary replication of avian H7 viruses, we inoculated mice with one of three viruses with differing PIs—A/mallard/Alberta/590/2010 (H7N8) (PI-4), A/mallard/MN/AI08-5469/2008 (H7N3) (PI-3), and A/ruddy turnstone/NJ/AI11-2098/2011 (H7N3) (PI-2)—and examined viral replication in different organs between 2 and 8 dpi. All three viruses replicated within the respiratory tract. Virus was detected in the nasal turbinates, trachea, and lung from 2 to 8 dpi, inclusive, and viral titers were similar among the three viruses (Table 4). Changes in the nasal cavities of mice inoculated with the viruses were minimal throughout the experimental period. By microscopy, changes in the nasal cavity involved only respiratory-type epithelium lining the turbinates and maxillary sinus and included areas of epithelial deciliation and attenuation, small foci of apoptosis/necrosis, and small amounts of serohemorrhagic fluid in the lumen. Tracheal changes were also mild and similar between viruses and were present as early as 2 dpi. The changes started as deciliation, attenuation, apoptosis, and small-group necrosis of epithelial cells that progressed to epithelial hyperplasia.
TABLE 4.
Avian H7 viral titers in the mouse respiratory tract
| Virus | Subtype | PI | Day | Virus detection ina: |
Lung viral titerb | |
|---|---|---|---|---|---|---|
| Nasal turbinate | Trachea | |||||
| Negative control | NAc | NA | 2 | − | − | ND |
| A/mallard/Alberta/590/2010 | H7N8 | 4 | 2 | + | + | 6.43 ± 0.06 |
| 4 | + | + | 6.21 ± 0.09 | |||
| 5 | + | + | 6.43 ± 0.06 | |||
| 6 | + | + | 6.2 | |||
| 7 | + | + | 5.03 | |||
| A/mallard/MN/AI08-5469/2008 | H7N3 | 3 | 2 | + | + | 5.84 ± 0.29 |
| 4 | + | + | 6.05 ± 0.29 | |||
| 6 | + | + | 6.39 ± 0.05 | |||
| 7 | + | + | 5.86 | |||
| 8 | + | + | 5.2 | |||
| A/ruddy turnstone/NJ/AI11-2098/2011 | H7N3 | 2 | 2 | + | + | 5.56 ± 0.29 |
| 4 | + | + | 5.47 ± 0.38 | |||
| 5 | + | + | 5.07 | |||
| 6 | + | + | 6.87 | |||
| 7 | + | + | 6.53 | |||
| 8 | + | + | 6.87 | |||
+, positive; −, negative. Samples were screened in 3 eggs and classified as positive if all three eggs were positive by hemagglutination.
Viral titers are expressed as EID50/ml ± standard error of the mean. At later time points few mice precluded calculation of the standard error of the mean. ND, not done.
NA, not applicable.
There was evidence of extrapulmonary spread of these viruses in mice. All three viruses were detected in the heart on all the days tested and were also found in the brain, although only A/mallard/Alberta/590/2010 (H7N8) (PI-4) was found in the brain on all the days tested (Table 5). In the heart, pathology was similar between viruses, as evident from rare to low numbers of small foci of myocardial fiber necrosis with minimal to moderate inflammation (neutrophils and/or lymphocytes), often with mild mineralization at 2 and 4 dpi.
TABLE 5.
Avian H7 virus replication in mouse extrapulmonary tissues
| Virus (PI) | Subtype | Day | Virus detection ina: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stomach | Duodenum | Jejunum | Ileum | Colon | Pancreas | Spleen | Liver | Heart | Brain | |||
| Negative control | NAb | 2 | − | − | − | − | − | − | − | − | − | − |
| A/ruddy turnstone/NJ/AI11-2098/2011 (H7N3) (PI-2) | H7N3 | 2 | + | + | − | − | − | − | − | + | + | + |
| 4 | − | − | − | − | − | − | − | − | + | + | ||
| 5 | − | − | − | − | + | − | − | − | + | − | ||
| 6 | − | − | − | − | − | − | − | + | + | − | ||
| 7 | − | + | − | − | − | − | − | − | + | + | ||
| 8 | − | − | − | − | − | − | − | − | + | + | ||
| A/mallard/MN/AI08-5469/2008 (H7N3) (PI-3) | H7N3 | 2 | − | − | − | − | − | − | − | + | + | − |
| 4 | − | − | − | − | + | − | − | − | + | + | ||
| 6 | − | − | − | − | − | − | − | − | + | + | ||
| 7 | − | − | + | − | − | − | − | − | + | − | ||
| 8 | + | − | + | − | − | + | − | − | + | + | ||
| A/mallard/Alberta/590/2010 (H7N8) (PI-4) | H7N8 | 2 | + | + | + | + | + | + | + | + | + | + |
| 4 | − | − | − | − | − | − | − | + | + | + | ||
| 5 | − | − | − | − | − | − | − | + | + | + | ||
| 6 | − | − | − | − | − | − | − | − | + | + | ||
| 7 | − | − | − | − | − | − | − | + | + | + | ||
+, positive; −, negative. Samples were screened in 3 eggs and classified as positive if all three eggs were positive by hemagglutination.
NA, not applicable.
Virus was also detected in the gastrointestinal tract, although the timing was different between viruses. A/mallard/MN/AI08_5469/2008 (H7N3) (PI-3) was found in the stomach and duodenum at 7 and 8 dpi and in the colon on day 4. However, A/ruddy turnstone/NJ/AI11_2098/2011 (H7N3) (PI-2) was found in the stomach and duodenum on day 2 and in the colon on day 5 and was again found in the duodenum on day 7. The stomachs of some animals inoculated with A/mallard/MN/AI08-5469/2008 (H7N3) (PI-3) and A/ruddy turnstone/NJ/AI11-2098/2011 (H7N3) (PI-2) showed mild changes, including mildly dilated glands, a slight increase in mitoses in the epithelium, and mild multifocal infiltrations of lymphocytes and neutrophils in the lamina propria.
A/mallard/Alberta/590/2010 (H7N8) (PI-4) was found in all organs tested at 2 dpi but was not detected in any organs, apart from the liver, heart, and brain, after 2 dpi (Table 5). While all the viruses tested were detected in some extrapulmonary tissues, only the PI-4 virus, A/mallard/Alberta/590/2010 (H7N8), showed such diffuse replication in the gastrointestinal tract, pancreas, and spleen. Of all the organs studied, the heart was the only organ where virus was detected on all days. No histopathological changes were observed in the brain, conjunctiva, liver, pancreas, duodenum, jejunum, ileum, colon, or spleen in mice inoculated with any of the viruses.
Low-pathogenic H7 viruses can replicate and cause pathology in the mouse lung.
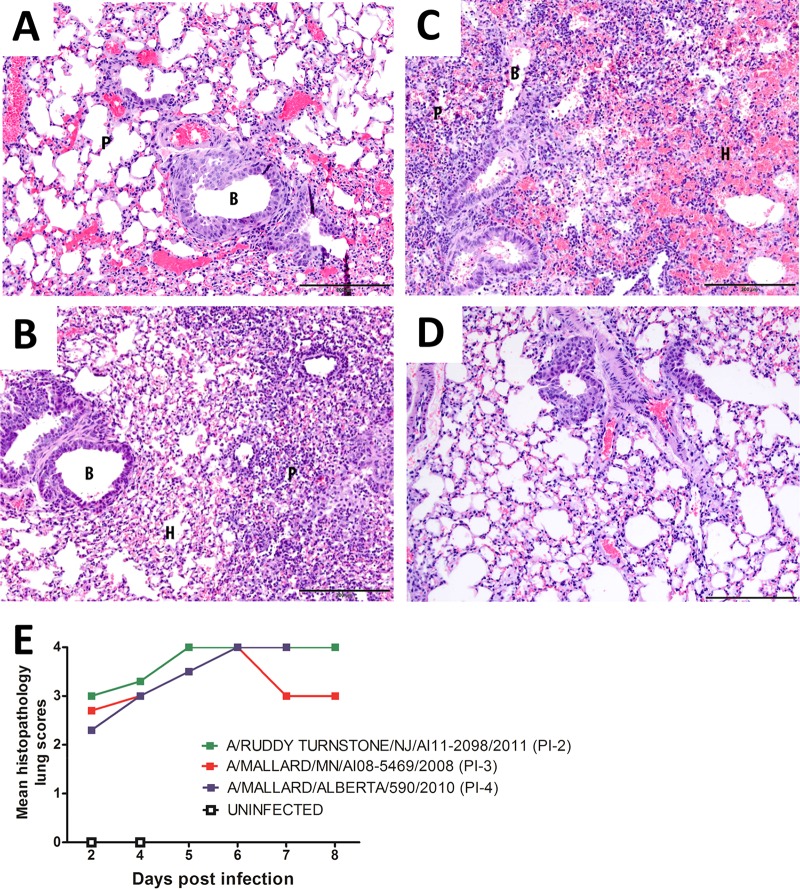
Pathology studies performed on the lungs of mice infected with these viruses revealed mild to moderate bronchointerstitial pneumonia beginning at 2 dpi and then progressing in severity, peaking by 5 or 6 dpi (Fig. 5). At 2 dpi, mice inoculated with any of the three viruses demonstrated similar changes in the lungs, characterized by severe bronchiolar epithelial hyperplasia with mild to moderate multifocal bronchiolar epithelial apoptosis and/or necrosis and mild to moderate peribronchiolar lymphocytic infiltration involving a small to moderate number of bronchioles. Multifocal interstitial involvement was mild and characterized by thickening of the alveolar septa and the presence of mononuclear cells in the alveoli. Mild to severe interstitial hemorrhage was also present. Over the next 6 days, in all groups, the changes progressed to more severe and extensive necrosis of the bronchiolar epithelium. Ulceration and changes in the interstitium became more widespread, with moderate numbers of lymphocytes and macrophages filling the alveoli. Hemorrhage continued to be a prominent feature. Overall, there were no obvious differences in lung pathology in mice inoculated with the PI-4, PI-3, or PI-2 viruses.
FIG 5.
Progression of bronchointerstitial pneumonia in the lungs of mice infected with A/mallard/MN/AI08_5469/2008 (H7N3). (A) At 2 dpi, the bronchiolar epithelium was hyperplastic, the interstitium was congested, and alveolar septa were mildly thickened. (B) At 4 dpi, the bronchiolar epithelium was still slightly hyperplastic. Portions of the parenchyma displayed thickened cellular alveolar septa and mild infiltration of lymphocytes and histiocytes into the alveoli. Small amounts of hemorrhage were also seen in alveoli. (C) At 6 dpi, there was severe bronchointerstitial pneumonia. Bronchioles were either hyperplastic or necrotic. The parenchyma showed large areas of hypercellularity due to thickened cellular septa and filling of alveoli with moderate numbers of lymphocytes and histiocytes. There were also large areas of hemorrhage. (D) Uninfected lung. Sections were stained with hematoxylin and eosin. B, bronchiole; P, parenchyma; H, areas of hemorrhage. Scale bars = 200 μm. (E) The development of histopathologic changes in the lungs of mice inoculated with viruses classified as PI-4, PI-3, and PI-2 was similar in severity and progression. Histopathologic changes were scored 0 to 4: 0, unremarkable; 1, minimal; 2, mild; 3, moderate; 4, severe.
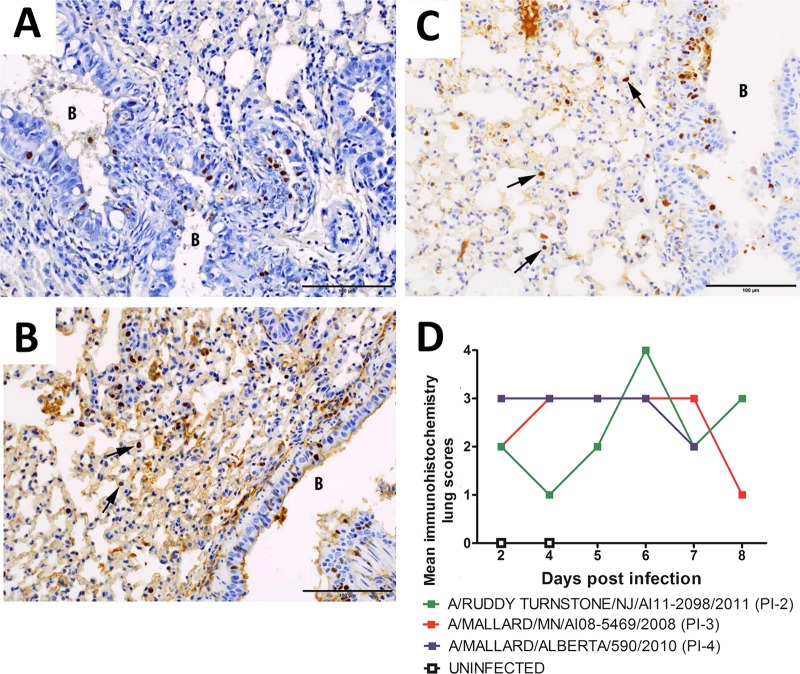
Viral antigen was detected by immunohistochemistry (IHC) in the lungs of mice infected with all three viruses (Fig. 6). As early as 2 dpi, moderate to large amounts of viral antigen were detected in the bronchiolar epithelium and a lesser amount in interstitial cells. With time, the amount of viral antigen decreased in the bronchiolar epithelium and increased in the interstitium in all groups. Antigen was still present at 8 dpi in mice inoculated with the PI-2 or PI-3 virus. Mice inoculated with the PI-4 virus did not survive to 8 dpi. The mean IHC scores were similar in mice inoculated with PI-4 or PI-3 viruses; however, scores were lower in mice inoculated with the PI-2 virus from 2 to 5 dpi (Fig. 6D).
FIG 6.
Progression of infection in the lungs of mice infected with A/mallard/MN/AI08_5469/2008 (H7N3). (A) Hyperplastic bronchiole at 2 dpi with many epithelial nuclei staining positive for influenza virus. (B) Epithelial cell nuclei in a bronchiole and nuclei of interstitial cells (the arrows point to two examples) both stained positive for influenza virus at 4 dpi. (C) Many epithelial cell nuclei in a bronchiole and multiple nuclei of interstitial cells (the arrows point to three examples) stained positive for influenza virus at 6 dpi. DAB chromogen was used to stain influenza virus-positive cells (brown), with hematoxylin as a counterstain (blue). B, bronchiole. Scale bars = 100 μm. (D) The amounts of influenza virus antigen detected in mice inoculated with viruses classified as PI-4, PI-3, and PI-2 were similar; however, less antigen was detected in mice inoculated with the PI-2 virus at 4 and 5 dpi than in mice inoculated with the PI-3 or PI-4 virus. The amount of influenza virus antigen detected by immunohistochemistry was scored subjectively 0 to 4: 0, none; 1, minimal; 2, mild; 3, moderate; and 4, severe.
Phylogenetic analysis revealed a possible relationship between low-pathogenic H7 avian influenza viruses and highly pathogenic H7 viruses based on the pathogenicity index.
We constructed neighbor-joining trees based on amino acid sequences to determine if there was any relationship between the viruses studied here and HPAI H7 viruses isolated during poultry outbreaks in the Americas. These outbreaks occurred in Mexico in 2012 (A/Mexico/InDRE7218/2012 [H7N3]), in Canada in 2007 (A/chicken/Saskatchewan [SK]/HR_00011/2007 [H7N3]), in Canada in 2004 (A/Canada/rv504/2004 [H7N3]), and in Chile in 2002 (A/chicken/Chile/4957/2002 [H7N3]).
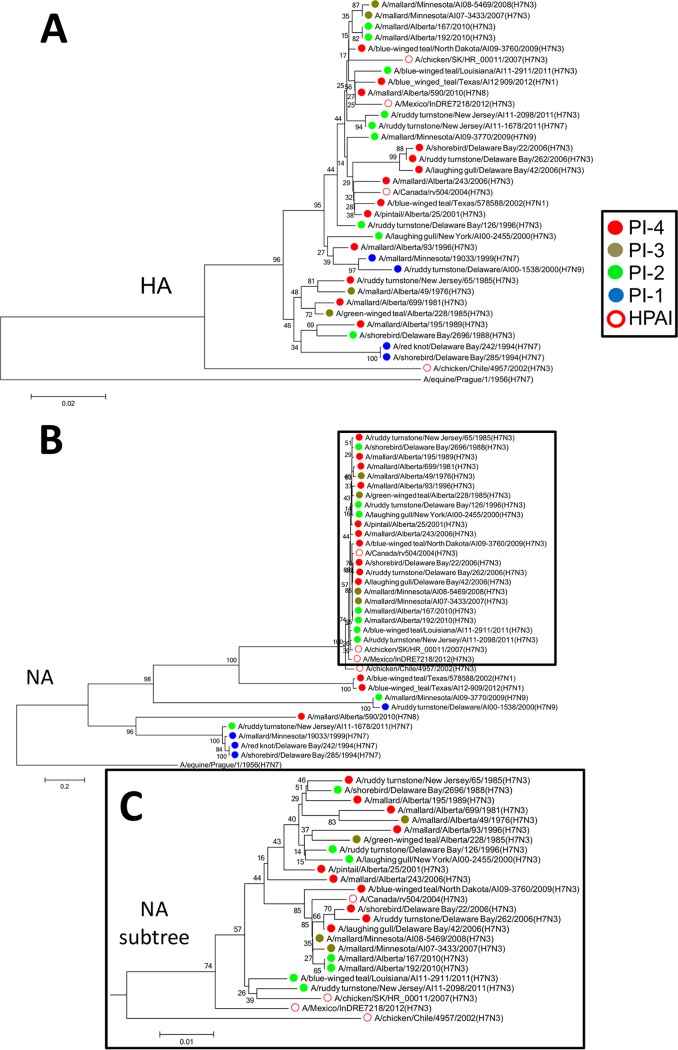
The hemagglutinins (HAs) of PI-4 viruses isolated between 2001 and 2006 clustered with the HA of A/Canada/rv504/2004 (H7N3), and the HAs of two PI-4 viruses and one PI-2 virus isolated between 2010 and 2012 clustered with the HA of A/Mexico/InDRE7218/2012 (H7N3). None of the HAs of these viruses were similar to that of A/chicken/Chile/4957/2002 (H7N3) (Fig. 7A). There was also clustering of the NAs of PI-4 viruses isolated between 2006 and 2009 with the NA of A/Canada/rv504/2004 (H7N3) (Fig. 7B and C).
FIG 7.
Phylogenetic analysis revealed similarities between the HAs and NAs based on the PI. (A) The HAs of PI-4 viruses isolated between 2001 and 2006 clustered with the HA of the HPAI virus A/Canada/rv504/2004 (H7N3), and the HAs of two PI-4 viruses and one PI-2 virus isolated between 2010 and 2012 clustered with the HA of the HPAI virus A/Mexico/InDRE7218/2012 (H7N3). None of the HAs of these viruses were similar to that of the HPAI virus A/chicken/Chile/4957/2002 (H7N3). (B) The NAs of PI-4 viruses isolated between 2006 and 2009 clustered with the NA of A/Canada/rv504/2004 (H7N3). No other obvious clustering was observed between the NAs of the viruses studied here and those of the HPAI viruses. (C) Detail of the subtree highlighted by a frame in panel B. The neighbor-joining trees were constructed with the full amino acid-coding sequence of each gene segment of the viruses using MEGA6 and rooted to sequences from A/equine/Prague/1/1956 (H7N7). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The evolutionary distances were computed using the Poisson correction method. The viruses were labeled according to their PI classifications.
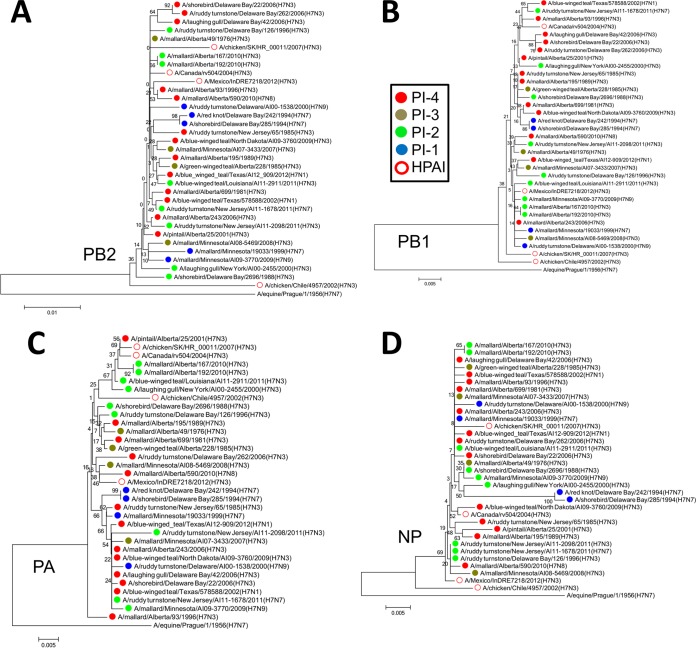
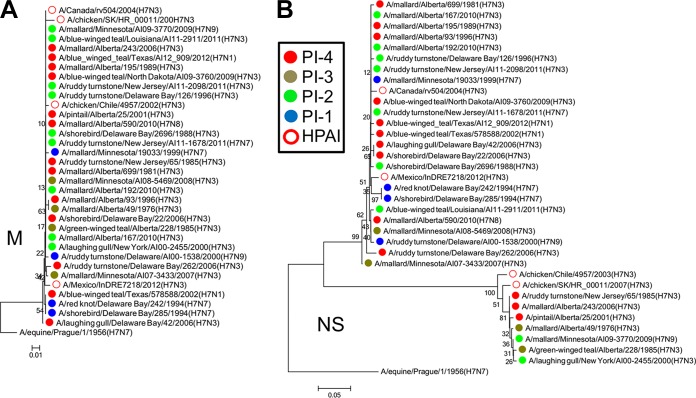
There were also similarities in the internal genes. The PB2s of A/Mexico/InDRE7218/2012 (H7N3), A/Canada/rv504/2004 (H7N3), and A/chicken/SK/HR_00011/2007 (H7N3) were similar (Fig. 8A). PB1 of A/Canada/rv504/2004 (H7N3) clustered with those of five PI-4 viruses isolated from 1996 to 2006 and one PI-2 virus isolated in 2011, suggesting that this PB1 may have originated from shorebirds or ducks and then persisted in this reservoir beyond the poultry outbreak (Fig. 8B). The PAs of A/Canada/rv504/2004 (H7N3) and A/chicken/SK/HR_00011/2007 (H7N3) clustered together, along with that of a PI-4 virus from 2001 and several related PI-2 viruses from 2000, 2010, and 2011. Interestingly, the PA of A/chicken/Chile/4957/2002 (H7N3) also clustered with these viruses. With the exception of M, which was similar in all these viruses (Fig. 9A), PA was the only segment in A/chicken/Chile/4957/2002 (H7N3) that clustered with any of these viruses. The PA of A/Mexico/InDRE7218/2012 (H7N3) also clustered with those of two PI-4 viruses and one PI-3 virus, isolated between 2006 and 2010 (Fig. 8C). The NPs of A/Mexico/InDRE7218/2012 (H7N3) and A/Canada/rv504/2004 (H7N3) also clustered with those of PI-4 viruses isolated between 1985 and 2010 (Fig. 8D).
FIG 8.
Phylogenetic analysis revealed similarities between PB2, PB1, PA, and NPs based on the PI. (A) The PB2s of the HPAI viruses A/Mexico/InDRE7218/2012 (H7N3), A/Canada/rv504/2004 (H7N3), and A/chicken/SK/HR_00011/2007 (H7N3) clustered with each other and with those of PI-4 viruses. (B) PB1 of the HPAI virus A/Canada/rv504/2004 (H7N3) clustered with those of five PI-4 viruses and one PI-2 virus. (C) The PAs of the HPAI viruses A/Canada/rv504/2004 (H7N3) and A/chicken/SK/HR_00011/2007 (H7N3) clustered together, along with a those of PI-4 virus and several related PI-2 viruses. The PA of the HPAI virus A/chicken/Chile/4957/2002 (H7N3) also clustered with these viruses. The PA of A/Mexico/InDRE7218/2012 (H7N3) clustered with those of two PI-4 viruses and one PI-3 virus. (D) The NPs of A/Mexico/InDRE7218/2012 (H7N3) and A/Canada/rv504/2004 (H7N3) clustered with those of PI-4 viruses. The neighbor-joining trees were constructed with the full amino acid-coding sequence of each gene segment of the viruses using MEGA6 and rooted to sequences from A/equine/Prague/1/1956 (H7N7). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The evolutionary distances were computed using the Poisson correction method. The viruses were labeled according to their PI classifications.
FIG 9.
Phylogenetic analysis revealed that the M proteins of the viruses were similar and that two distinct groups of NS proteins were circulating. (A) The M proteins of all the viruses were similar. (B) Two clear branches were evident in the NS tree: allele A and allele B. The allele B branch contained the HPAI virus A/chicken/SK/HR_00011/2007 (H7N3) clustered with viruses with differing PIs. These NSs were also more similar to that of the HPAI virus A/chicken/Chile/4957/2002 (H7N3) than to those of the other viruses. The neighbor-joining trees were constructed with the full amino acid-coding sequence of each gene segment of the viruses using MEGA6 and rooted to sequences from A/equine/Prague/1/1956 (H7N7). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The evolutionary distances were computed using the Poisson correction method. The viruses were labeled according to their PI classifications.
There were two clear branches in the nonstructural (NS) gene tree, allele A and allele B (Fig. 9B). The allele B branch contained A/chicken/SK/HR_00011/2007 (H7N3) clustered with PI-4, PI-3, and PI-2 viruses isolated between 1976 and 2009, indicating that this NS may have originated from shorebirds or ducks and then persisted beyond the poultry outbreak. These NSs were also more similar to that of A/chicken/Chile/4957/2002 (H7N3) (Fig. 9B). Overall, it appears that the circulation of the more pathogenic viruses was somewhat coincidental with poultry outbreaks in North America and that these segments persisted after the outbreaks.
H7 viruses showed strong binding preferences for avian-like α2,3-linked sialic acids.
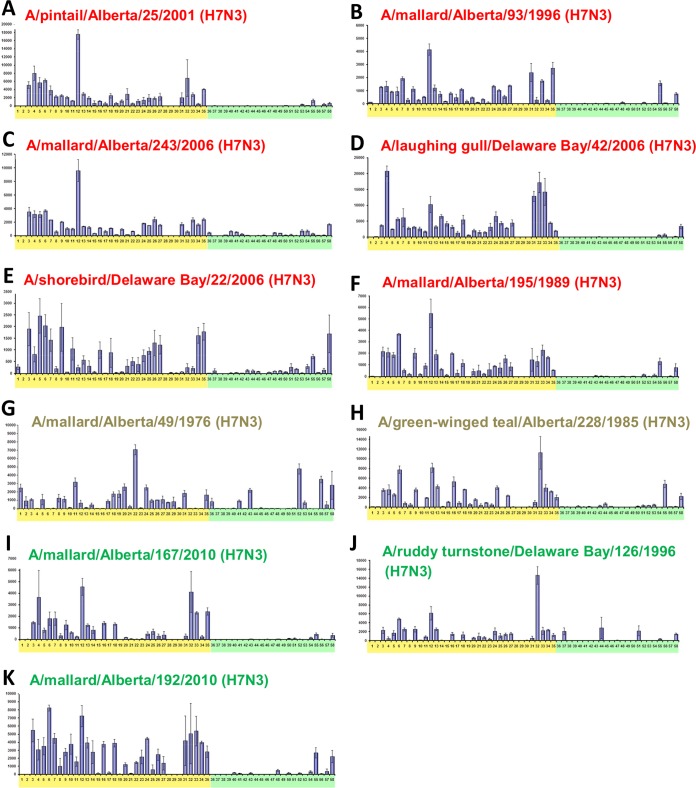
As we were interested in the potential of the AIVs to cause disease in mammals, we performed glycan binding analysis on PI-4, PI-3, and PI-2 H7N3 viruses to determine if they bound to mammalian-like α2,6-linked sialic acids or avian-like α2,3-linked sialic acids. We were also interested in any possible trends over the 30-year time frame during which we isolated the viruses. All the viruses showed similar binding preferences for avian-like α2,3-linked sialic acids, and this specificity was fairly consistent over time (Fig. 10). Interestingly, there was some recognition of mammalian-like α2,6-linked sialic acids, most notably by A/shorebird/DE/22/2006 (H7N3) (PI-4) and A/mallard/Alberta/49/1976 (H7N3) (PI-3) (Fig. 10E and G, respectively). In summary, the receptor binding specificities of these viruses generally showed a preference for α2,3-linked sialic acids, and this preference appeared to be stable over the time frame studied. Further, there was no link between the sialic acid binding preference and the PI. However, the sialic acid binding preference has previously been shown not to be predictive of pathogenicity in mammals. This is particularly evident in the case of H5N1 viruses (28–31).
FIG 10.
H7N3 viruses showed strong binding preferences for α2,3-linked sialic acids. Glycan binding arrays were comprised of α2,3-linked sialic acids (yellow shading) and α2,6-linked sialic acids (green shading). H7N3 viruses classified as PI-4 (A to F; red text), PI-3 (G and H; beige text), and PI-2 (I to K; green text), which caused 100%, 100%, and 80% mortality in mice, respectively, all showed strong preference for α2,3-linked sialic acids. There was some recognition of mammalian-like α2,6-linked sialic acids, particularly by A/shorebird/Delaware Bay/22/2006 (H7N3) (E) and A/mallard/Alberta/49/1976 (H7N3) (G). There were no indications that the binding preferences changed over time. Error bars indicate SEM.
DISCUSSION
AIVs of the H7 subtype of both low and high pathogenicity are of concern from both an agricultural and a public health viewpoint. These viruses have been responsible for numerous outbreaks that caused severe and often lethal infections in poultry, resulting in the destruction of millions of birds with significant economic impact. Human infections have also been associated with these outbreaks. Wild birds and ducks are important reservoirs for H7 viruses, yet little is known about the potential of these viruses to cause disease in mammals. As such, our goal was to study the potential of the viruses to cause disease in mammals. The viruses we studied were isolated during our continuing surveillance of wild birds in the United States and Canada from the 1970s to the 2010s.
We used the DBA/2J mouse model to assess the pathogenicity of the viruses in mammals. This model is advantageous, as DBA/2J mice are susceptible to influenza virus infection, thereby removing the requirement for viral adaptation present with other mouse strains (32). We previously used the DBA/2J mouse model to study the pathogenicity of H1N1 AIVs and found it useful (33). In our previous study, 100% mortality was observed in 97% of mice inoculated with H1N1 AIVs (33). In contrast, preliminary studies with H2, H3, H4, H6, H10, and H12 IAVs revealed that only an H6N2 and an H10N4 IAV caused 100% mortality. Further, the overall mortality rates were much lower, with 12 of 18 (67%) AIVs causing 0% to 40% mortality (33). Here, we show that 27 of 30 (90%) LP H7 AIVs were capable of causing mortality in mice without prior adaptation (Table 1). Of the viruses studied here, those of duck origin were more pathogenic overall than those of shorebird origin (Fig. 1 and 2). This difference was not obvious in our previous work with H1N1 AIVs (33). However, a limiting factor of that study was that shorebird origin H1N1 IAVs were underrepresented, as only 6 of the 31 H1N1 IAVs were of shorebird origin. It would be interesting to conduct further studies to determine if there are specific adaptations that support our observation that duck origin H7 AIVs appear more pathogenic than shorebird origin AIVs. Overall, it appears that, like H1N1 AIVs, H7 AIVs are also capable of pathogenicity in the mouse model without prior adaptation.
There did not appear to be any restriction to the pathogenicity of H7 AIVs based on NA subtype, further confirming that H7 viruses are less restricted in the subtypes that can infect and cause disease in mammals than in other subtypes, such as H5 and H9 (Fig. 3). The majority of the viruses tested here were H7N3, reflecting the prevalence of this subtype in nature, though the reason for the apparent dominance of H7N3 in nature is unclear (7).
It was interesting that, despite pathogenicity differences, there were no obvious differences in lung pathology between the PI-4, PI-3, and PI-2 viruses (Fig. 5). This was somewhat unexpected, as lung pathology is an important cause of mortality from influenza virus infection in mice. However, IHC staining of the lung indicated that the replication and/or spread of the PI-2 virus in the lung was slower than in the PI-3 and PI-4 viruses (Fig. 6). It would be interesting to investigate this further using a larger cohort of viruses.
Despite these viruses being LP and lacking multibasic cleavage sites, they could spread beyond the respiratory tract. Extrapulmonary spread seemed more prominent in PI-4 viruses as, unlike PI-2 and PI-3 IAVs, the PI-4 IAV was detected in all organs tested at 2 dpi (Table 3). From 4 dpi on, the PI-4 IAV was not detected in the gastrointestinal tract, the pancreas, or the spleen, although it was still present in the liver, heart, and brain during this time. Therefore, it appeared that widespread systemic spread of the PI-4 IAV occurred early in infection and became restricted as time progressed. All three viruses described in Table 5 were also detected at various times in the gastrointestinal tract. Unlike the brain, there did not appear to be any link between the PI and virus detection in the intestine (Table 2). There also did not appear to be a link between the PI and viral shedding in feces, as fecal shedding was observed from mice inoculated with viruses from all PIs at 2 dpi (Table 3). This is similar to the results obtained studying H1N1 IAVs in DBA/2J mice (33). Overall, it was somewhat surprising that these AIVs spread so readily beyond the respiratory tract, particularly as they were LP. It would be interesting to extend these studies into other mammalian models to further confirm these observations.
We previously reported that the sequences of the HPAI H7 viruses isolated during the poultry outbreaks were most closely related to those of LP H7 viruses isolated in the same year and/or in years immediately preceding the outbreak (7). In this study, we sought to determine if there were similarities between the HPAI H7 viruses and the PI-4 viruses. It was interesting that some gene segments of the HPAI viruses from outbreaks in Canada and Mexico clustered with the PI-4 viruses isolated around the same time the outbreaks occurred. Some of these PI-4 viruses were isolated just prior to, and just after, the relevant poultry outbreaks. However, the limited number of sequences restricted the power of the analysis, and as such, it was not possible to draw definitive conclusions. Nevertheless, this raised the question of whether PI-4 viruses are more likely to cause poultry outbreaks and become HPAI viruses.
In conclusion, we surveyed 30 North American H7 AIVs isolated over a 30-year period. Of these viruses, 76.7% were highly pathogenic in the DBA/2J mouse model, as they caused 80% or greater mortality. Therefore, LP H7 AIVs are able to infect and cause disease in the mouse model without prior adaptation, indicating that the viruses can infect and cause disease in mammals without prior adaptation. Further studies to determine the pathogenicity of the viruses in other mammalian models of influenza are warranted. This study again highlights the importance of the wild bird reservoir as a source of potentially pathogenic influenza viruses and the necessity for ongoing surveillance of this reservoir and continued characterization and monitoring of the viruses present.
MATERIALS AND METHODS
Ethics statement.
Animal experiments were conducted in animal biosafety level 2+ facilities at St. Jude Children's Research Hospital and the University of Georgia (UGA). Animal experiments involving A/Anhui/1/2013 (H7N9) were conducted in an animal biosafety level 3+ facility at St. Jude Children's Research Hospital. All animal experiments were conducted in compliance with the policies of the National Institutes of Health and the Animal Welfare Act and with the approval of the St. Jude Children's Research Hospital institutional and the UGA animal care and use committees (protocol number 081; approval date, 31 July 2014).
Viruses.
All the AIVs included in this study were originally isolated from swab samples or feces from North American ducks, shorebirds, and gulls from 1976 to 2011. The viruses were propagated in 10-day-old embryonated chicken eggs, and viral titers were measured by titration in eggs to determine the EID50. Mice were inoculated with viruses that had been minimally passaged—no more than three times—in eggs. Genomic sequencing of the viruses was performed by the J. Craig Venter Institute (JCVI).
Animals.
Six-week-old female DBA/2J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Upon arrival, all the mice were acclimatized for 1 week prior to infection. Food and water were available ad libitum.
Pathogenicity screening.
Groups of five mice were anesthetized by intraperitoneal injection of avertin at a dose of 275 μl in mice weighing ≥13.5 g or 250 μl in mice weighing <13.5 g prior to intranasal inoculation with 106 EID50 of virus in a 30-μl volume. An uninfected group was anesthetized and intranasally administered 30 μl of phosphate-buffered saline, pH 7.2 (PBS). Mouse cages were placed on heating pads over medium heat while the mice recovered from anesthesia. Weights and body conditioning scores were taken daily for 14 dpi according to the Guidelines for Assessing the Health and Condition of Mice (34). If ≥25% weight loss was observed, the mice were euthanized using CO2 and cervical dislocation.
PIs were assigned based on total pathogenicity scores, which were the sum of the survival and weight loss scores {survival score = 0.8 × (survival area under the curve [AUC]/maximum AUC); weight loss score = 0.2 × (weight loss AUC/maximum AUC)}. The mean weight loss of groups of five mice, each group inoculated with a different virus, was used in these calculations. As mortality was more important than morbidity in our screening, we used 80% weight for survival and 20% weight for weight loss after normalizing the AUCs to the maximum AUC that could be obtained with no mortality or morbidity (the uninfected group). The AUCs for survival and weight loss were calculated using GraphPad Prism.
Pathogenesis study.
Groups of 15 mice were inoculated with 106 EID50 of the PI-4 virus A/ruddy turnstone/NJ/AI11-2098/2011 (H7N3), A/mallard/MN/AI08-5469/2008 (H7N3), or A/mallard/Alberta/590/2010 (H7N8) intranasally, as described above. Three mice were also inoculated with PBS and euthanized at 2 dpi. Three mice per group were euthanized using CO2 at 2, 4, 6, and 8 dpi. If ≥25% weight loss was observed, mice were euthanized on that day. Tissues were fixed in formalin for pathology and immunohistochemistry studies.
Sample collection and virus titration.
Fecal samples were collected at 2, 3, and 6 dpi. One fresh specimen per mouse was collected, and the samples were pooled on each collection day, resulting in one pooled sample per cage. The samples were stored at 4°C overnight. For virus isolation, 1 ml of isolation medium (PBS plus glycerol with antibiotics) was added to each sample and vortex mixed. Any viruses present were propagated in 10-day-old embryonated chicken eggs, and viral titers were determined by EID50.
Lungs were collected from mice postmortem, homogenized in 0.5 ml PBS containing antibiotics, and clarified by centrifugation, and 0.1 ml was injected into each of three 10-day-old embryonated chicken eggs, which were incubated at 35°C for 48 h and then chilled to 4°C overnight. Virus was detected by hemagglutination assay. Positive tissues were titrated to determine EID50.
Extrapulmonary tissues were collected from mice in the pathogenicity study postmortem, homogenized in 0.5 ml PBS containing antibiotics, and clarified by centrifugation, and 0.1 ml was injected into each of three 10-day-old embryonated chicken eggs, which were incubated at 35°C for 48 h and then chilled to 4°C overnight. Virus was detected by hemagglutination assay. Samples were classified as positive if all three eggs were positive by hemagglutination.
Histopathology.
Tissues were collected during the pathogenicity study on the days indicated, fixed in 10% buffered formalin, processed, and embedded in paraffin. Four-micrometer sections were cut and stained with hematoxylin and eosin and by immunohistochemistry (one mouse per group) for influenza A viral antigen. Immunohistochemistry was conducted on sections that were deparaffinized and rehydrated through graded alcohols, followed by enzymatic antigen retrieval (proteinase K; Dako), quenching of endogenous peroxidases with H2O2, and blocking with a commercial protein-blocking reagent (Power Block; Dako). The primary antibody was either a mouse monoclonal antibody to influenza A virus (1:250; Meridian) or goat polyclonal antibody (1:3,000; Meridian). For detection, appropriate biotinylated secondary antibodies (Vector) were followed by horseradish peroxidase-labeled streptavidin (Biocare) and diaminobenzidine (DAB) (Dako).
Lung changes were scored 0 to 4 as follows: 0 was defined as unremarkable; 1 was defined as minimal changes in the bronchiolar epithelium with minimal peribronchiolar/perivascular inflammation; 2 was defined as mild multifocal bronchiolar epithelial changes with perivascular and peribronchiolar inflammation; 3 was defined as moderate multifocal bronchiolar epithelial changes with perivascular, peribronchiolar, and mild to moderate alveolar inflammation; and 4 was defined as marked diffuse bronchiolar epithelial changes with perivascular and peribronchiolar and marked alveolar inflammation. Immunohistochemistry on the lung was scored subjectively as 0 to 4 (0, no staining, to 4, severe).
Phylogenetic analysis.
Neighbor-joining trees were constructed with the full amino acid-coding sequences of each gene segment of the viruses using MEGA6 (Megasoftware). The trees were rooted to sequences from A/equine/Prague/1/1956 (H7N7). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The evolutionary distances were computed using the Poisson correction method.
Glycan binding.
The glycan list used in this study was as previously described (35). It was a custom array consisting of 58 glycans produced by the Paulson group (Scripps Research Institute, La Jolla, CA, USA). Viruses used on glycan binding arrays were inactivated using 0.1% (vol/vol) β-propriolactone. Inactivated virus was added to the array and incubated in a humidified chamber protected from light for 1 h at room temperature. The virus was then removed, and the array was washed 3 times with PBS plus 0.05% Tween, pH 7.4. The arrays were then dipped 3 times in PBS and then 3 times in distilled H2O. The slides were dried by centrifugation and scanned using a ProScanArray Express HT (PerkinElmer) confocal slide scanner for Alexa Fluor 488. Signal data were collected using Imagene (BioDiscovery), and the signal data were processed to determine average (mean signal minus mean background) values of 4 replicate spots on the array for each unique printed glycan.
ACKNOWLEDGMENTS
We thank the JCVI team of researchers who provided technical expertise in support of the viral sequencing and assembly pipeline for this project, especially Rebecca Halpin, Timothy Stockwell, Nadia Fedorova, and Susmita Shrivastava.
This study was funded by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health under contract no. HHSN272200900007C, HHSN266200700005C, and HHSN272201400006C.
REFERENCES
- 1.Abdelwhab EM, Veits J, Mettenleiter TC. 2014. Prevalence and control of H7 avian influenza viruses in birds and humans. Epidemiol Infect 142:896–920. doi: 10.1017/S0950268813003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freidl GS, Meijer A, de Bruin E, de Nardi M, Munoz O, Capua I, Breed AC, Harris K, Hill A, Kosmider R, Banks J, von Dobschuetz S, Stark K, Wieland B, Stevens K, van der Werf S, Enouf V, van der Meulen K, Van Reeth K, Dauphin G, Koopmans M, FLURISK Consortium. 2014. Influenza at the animal-human interface: a review of the literature for virological evidence of human infection with swine or avian influenza viruses other than A(H5N1). Euro Surveill 19:20793. doi: 10.2807/1560-7917.ES2014.19.18.20793. [DOI] [PubMed] [Google Scholar]
- 3.Gibson CA, Daniels RS, Oxford JS, McCauley JW. 1992. Sequence analysis of the equine H7 influenza virus haemagglutinin gene. Virus Res 22:93–106. doi: 10.1016/0168-1702(92)90037-A. [DOI] [PubMed] [Google Scholar]
- 4.Kwon TY, Lee SS, Kim CY, Shin JY, Sunwoo SY, Lyoo YS. 2011. Genetic characterization of H7N2 influenza virus isolated from pigs. Vet Microbiol 153:393–397. doi: 10.1016/j.vetmic.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Webster RG, Hinshaw VS, Bean WJ, Van Wyke KL, Geraci JR, St Aubin DJ, Petursson G. 1981. Characterization of an influenza A virus from seals. Virology 113:712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- 6.Perroncito E. 1878. Epizoozia tifoide nei gallinacei. Annali Accad Agri Torino 21:87–126. [Google Scholar]
- 7.Krauss S, Stucker KM, Schobel SA, Danner A, Friedman K, Knowles JP, Kayali G, Niles LJ, Dey AD, Raven G, Pryor P, Lin X, Das SR, Stockwell TB, Wentworth DE, Webster RG. 2015. Long-term surveillance of H7 influenza viruses in American wild aquatic birds: are the H7N3 influenza viruses in wild birds the precursors of highly pathogenic strains in domestic poultry? Emerg Microbes Infect 4:e35. doi: 10.1038/emi.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLay PD, Casey HL, Tubiash HS. 1967. Comparative study of fowl plague virus and a virus isolated from man. Public Health Rep 82:615–620. doi: 10.2307/4593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, Meijer A, van Steenbergen J, Fouchier R, Osterhaus A, Bosman A. 2004. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 11.Subbarao K, Katz J. 2000. Avian influenza viruses infecting humans. Cell Mol Life Sci 57:1770–1784. doi: 10.1007/PL00000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.USDA. 22 November 2016. USDA confirms highly pathogenic H7N8 avian influenza in a commercial turkey flock in Dubois County, Indiana. USDA, Washington, DC: https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/newsroom-2016/newsroom-january-2016/ct-hpai-indiana-turkeys. [Google Scholar]
- 13.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc Natl Acad Sci U S A 105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambaryan AS, Matrosovich TY, Philipp J, Munster VJ, Fouchier RA, Cattoli G, Capua I, Krauss SL, Webster RG, Banks J, Bovin NV, Klenk HD, Matrosovich MN. 2012. Receptor-binding profiles of H7 subtype influenza viruses in different host species. J Virol 86:4370–4379. doi: 10.1128/JVI.06959-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belser JA, Davis CT, Balish A, Edwards LE, Zeng H, Maines TR, Gustin KM, Martinez IL, Fasce R, Cox NJ, Katz JM, Tumpey TM. 2013. Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J Virol 87:5746–5754. doi: 10.1128/JVI.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belser JA, Bridges CB, Katz JM, Tumpey TM. 2009. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis 15:859–865. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi L, Pujanauski LM, Davis AS, Schwartzman LM, Chertow DS, Baxter D, Scherler K, Hartshorn KL, Slemons RD, Walters KA, Kash JC, Taubenberger JK. 2014. Contemporary avian influenza A virus subtype H1, H6, H7, H10, and H15 hemagglutinin genes encode a mammalian virulence factor similar to the 1918 pandemic virus H1 hemagglutinin. mBio 5:e02116. doi: 10.1128/mBio.02116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison J, Josset L, Tchitchek N, Chang J, Belser JA, Swayne DE, Pantin-Jackwood MJ, Tumpey TM, Katze MG. 2014. H7N9 and other pathogenic avian influenza viruses elicit a three-pronged transcriptomic signature that is reminiscent of 1918 influenza virus and is associated with lethal outcome in mice. J Virol 88:10556–10568. doi: 10.1128/JVI.00570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, Wang D, Kelvin DJ, Li L, Zheng Z, Yoon SW, Wong SS, Farooqui A, Wang J, Banner D, Chen R, Zheng R, Zhou J, Zhang Y, Hong W, Dong W, Cai Q, Roehrl MH, Huang SS, Kelvin AA, Yao T, Zhou B, Chen X, Leung GM, Poon LL, Webster RG, Webby RJ, Peiris JS, Guan Y, Shu Y. 2013. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs. Science 341:183–186. doi: 10.1126/science.1239844. [DOI] [PubMed] [Google Scholar]
- 20.Qi X, Qian YH, Bao CJ, Guo XL, Cui LB, Tang FY, Ji H, Huang Y, Cai PQ, Lu B, Xu K, Shi C, Zhu FC, Zhou MH, Wang H. 2013. Probable person to person transmission of novel avian influenza A (H7N9) virus in Eastern China, 2013: epidemiological investigation. BMJ 347:f4752. doi: 10.1136/bmj.f4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. 2013. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Shi J, Deng G, Guo J, Zeng X, He X, Kong H, Gu C, Li X, Liu J, Wang G, Chen Y, Liu L, Liang L, Li Y, Fan J, Wang J, Li W, Guan L, Li Q, Yang H, Chen P, Jiang L, Guan Y, Xin X, Jiang Y, Tian G, Wang X, Qiao C, Li C, Bu Z, Chen H. 2013. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341:410–414. doi: 10.1126/science.1240532. [DOI] [PubMed] [Google Scholar]
- 23.Lam TT, Zhou B, Wang J, Chai Y, Shen Y, Chen X, Ma C, Hong W, Chen Y, Zhang Y, Duan L, Chen P, Jiang J, Zhang Y, Li L, Poon LL, Webby RJ, Smith DK, Leung GM, Peiris JS, Holmes EC, Guan Y, Zhu H. 2015. Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature 522:102–105. doi: 10.1038/nature14348. [DOI] [PubMed] [Google Scholar]
- 24.Blanchfield K, Kamal RP, Tzeng WP, Music N, Wilson JR, Stevens J, Lipatov AS, Katz JM, York IA. 2014. Recombinant influenza H7 hemagglutinins induce lower neutralizing antibody titers in mice than do seasonal hemagglutinins. Influenza Other Respir Viruses 8:628–635. doi: 10.1111/irv.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couch RB, Patel SM, Wade-Bowers CL, Nino D. 2012. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One 7:e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudenko L, Isakova-Sivak I, Donina S. 2013. H7N3 live attenuated influenza vaccine has a potential to protect against new H7N9 avian influenza virus. Vaccine 31:4702–4705. doi: 10.1016/j.vaccine.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, Lamirande EW, Jin H, Coelingh KL, Murphy BR, Kemble G, Subbarao K. 2009. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a phase I trial in healthy adults. Vaccine 27:3744–3753. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha DQ, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driskell EA, Pickens JA, Humberd-Smith J, Gordy JT, Bradley KC, Steinhauer DA, Berghaus RD, Stallknecht DE, Howerth EW, Tompkins SM. 2012. Low pathogenic avian influenza isolates from wild birds replicate and transmit via contact in ferrets without prior adaptation. PLoS One 7:e38067. doi: 10.1371/journal.pone.0038067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones JC, Baranovich T, Marathe BM, Danner AF, Seiler JP, Franks J, Govorkova EA, Krauss S, Webster RG. 2014. Risk assessment of H2N2 influenza viruses from the avian reservoir. J Virol 88:1175–1188. doi: 10.1128/JVI.02526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maines TR, Chen LM, Van Hoeven N, Tumpey TM, Blixt O, Belser JA, Gustin KM, Pearce MB, Pappas C, Stevens J, Cox NJ, Paulson JC, Raman R, Sasisekharan R, Katz JM, Donis RO. 2011. Effect of receptor binding domain mutations on receptor binding and transmissibility of avian influenza H5N1 viruses. Virology 413:139–147. doi: 10.1016/j.virol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boon AC, deBeauchamp J, Krauss S, Rubrum A, Webb AD, Webster RG, McElhaney J, Webby RJ. 2010. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model. J Virol 84:7662–7667. doi: 10.1128/JVI.02444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocer ZA, Krauss S, Stallknecht DE, Rehg JE, Webster RG. 2012. The potential of avian H1N1 influenza A viruses to replicate and cause disease in mammalian models. PLoS One 7:e41609. doi: 10.1371/journal.pone.0041609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foltz CJ, Ullman-Cullere M. 1999. Guidelines for assessing the health and condition of mice. Lab Animal 28:28–32. [PubMed] [Google Scholar]
- 35.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A 101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]