FIG 7.
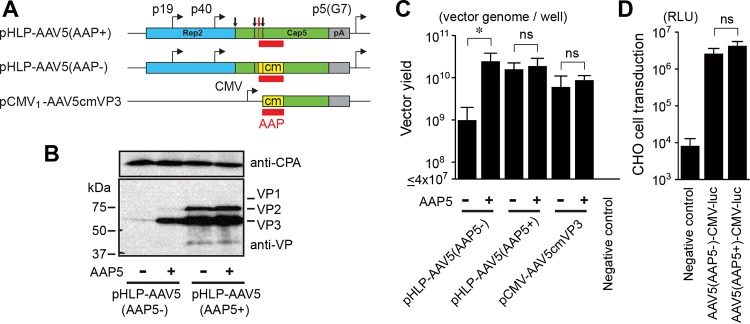
Production and characterization of AAV5 VP1/VP2/VP3 particles produced with and without AAP5. (A) Schematic representation of plasmids expressing AAV5 VP proteins. These plasmids were used for the experiments shown in panels B to D. The VP1, VP2, and VP3 translation start sites are indicated with black vertical lines and arrows from left to right. The AAP translation start site is indicated with red vertical lines and a red arrow. The AAP ORF is shown with red boxes. The codon-modified region is indicated with yellow boxes. pA, polyadenylation signal; p5(G7), AAV2 p5 promoter with the TATA box sequence TATTTAA replaced with GGGGGGG. (B) HEK 293 cells were transiently transfected with an AAV5 helper plasmid, pHLP-AAV5(AAP5+) or pHLP-AAV5(AAP5−), with or without a plasmid expressing AAP5. All the groups were also transfected with an adenovirus helper plasmid, pHelper, to induce protein expression from the AAV5 helper plasmids. At 48 h posttransfection, the AAV5 VP1, VP2, and VP3 proteins were probed with anti-AAV VP antibody (B1) by Western blotting. Cyclophilin A (CPA) was used as a loading control. (C) HEK 293 cells were transfected with the plasmids indicated to produce AAV5 VP1/VP2/VP3 particles or VP3-only particles containing a double-stranded AAV-CMV-GFP vector genome in the presence or absence of AAP5 expressed from a separate plasmid, pCMV3-FLAG-cmAAP5. At 5 days posttransfection, the medium and cells were harvested, and Benzonase-resistant viral genomes in each sample were quantified by a dot blot assay in an experiment performed in biological triplicates. The y axis shows the AAV vector titers (vector genomes) per well in a 6-well-plate format. (D) CHO-K1 cells were infected with either the AAV5(AAP5+)-CMV-luc or AAV5(AAP5−)-CMV-luc vector, which was produced with or without AAP5, respectively, at an MOI of 106. Luciferase activity was measured at 46 h postinfection in an experiment performed in biological triplicates. The negative-control group received the luciferase-containing samples prepared in the same manner except for the absence of AAV5 VP protein expression, which provides a measure of pseudotransduction. For the pseudotransduction control, the same sample volume as that for the AAV5(AAP5−)-CMV-luc vector preparation was used. The y axis shows relative light units (RLUs). Error bars represent standard deviations. An asterisk indicates statistical significance with a P value of <0.05 (two-tailed Welch's t test). ns, not significant.