FIG 3.
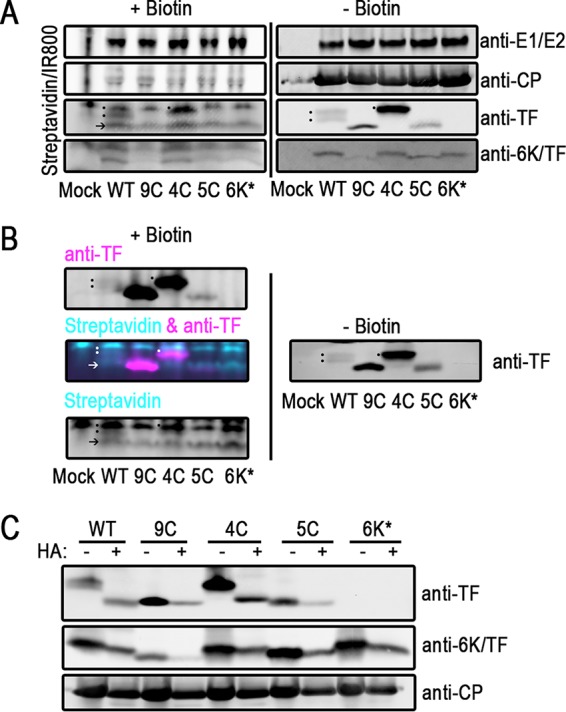
TF is palmitoylated on Cys residues in its shared N terminus. (A and B) Infected BHK cells were labeled with palmitic azide from 12 to 16 hpi. After harvesting of the cell lysate, proteins modified with palmitic azide were labeled via click chemistry with biotin. Proteins were separated by SDS-PAGE and transferred onto blotting paper. Biotinylated proteins were detected with fluorophore-conjugated streptavidin. Virus proteins were detected with specific antibodies. (A) On the same gel, untreated and biotinylated lysates were separated and detected. At the right are untreated virus proteins. On the left are biotin-conjugated palmitoylated proteins from the same lysates. The dots denote the palmitoylation signal from TF. The arrow indicates a host protein that is palmitoylated in virus infections, also indicated by an arrow in panel B (bottom). These experiments were repeated at least three times. (B) Click-modified samples were probed by using dual-color detection. Cyan indicates biotinylated protein, and magenta indicates virus protein. At the top left is the TF channel alone. At the bottom left is the streptavidin channel alone. At the middle left are overlaid TF and streptavidin channels. At the right, the same samples that were left untreated were electrophoresed on the same gel and detected only by anti-TF antibody. These experiments were repeated at least three times. (C) Infected BHK lysates were treated with hydroxylamine (HA) to remove palmitoyl moieties. Virus proteins were detected by Western blotting. One representative blot from at least three repetitions is shown.
