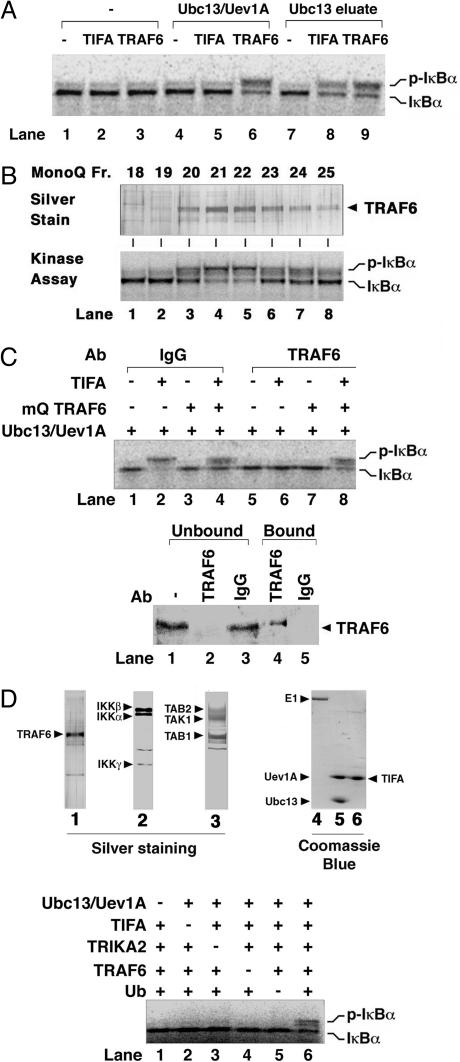
Fig. 2.
TIFA activates IKK through Ubc13–Uev1A and TRAF6. (A) Activation of IKK by TIFA requires Ubc13–Uev1A and another Ubc13-associated factor. HeLa S100 was incubated with Ubc13–Sepharose to remove endogenous Uev1A and other proteins that bound to the resin. The supernatant was used as “Ubc13-depleted extract” for IKK assays in the presence of recombinant TRAF6 (lanes 3, 6, and 9) or TIFA (lanes 2, 5, and 8). In lanes 4–6, recombinant Ubc13–Uev1A protein was added to the reaction, whereas in lanes 7–9, protein that bound to the Ubc13 column was eluted (Ubc13 eluate) and added to the assays. (B) Identification of TRAF6 as a Ubc13-interacting protein that is required for IKK activation by TIFA. Endogenous TRAF6 protein was purified as described in ref. 16, and fractions from the last MonoQ step were analyzed by silver staining (Upper) or IKK kinase assays (Lower). (C) TRAF6 is required for IKK activation by TIFA in vitro. HeLa S100 was subjected to immunoprecipitation with an antibody against TRAF6 (lanes 5–8) or a control IgG (lanes 1–4). The supernatants were then analyzed by immunoblotting with a TRAF6 antibody (Lower) or by IKK assays (Upper) in the presence of Ubc13–Uev1A, TIFA, and purified endogenous TRAF6, as indicated. (D) (Upper) Silver or Coomassie blue staining of purified proteins. TRAF6 (native, purified from HeLa S100; lane 1), IKK (native, purified from HeLa S100; lane 2), TRIKA2 (recombinant, purified from Sf9; lane 3), E1 (recombinant, purified from Sf9; lane 4), Ubc13–Uev1A (recombinant, purified from E. coli; lane 5), and TIFA (recombinant, purified from E. coli; lane 6). (Lower) Reconstitution of TIFA-induced IKK pathway in vitro. Purified native IKK complex and TRAF6 were incubated with recombinant proteins of TIFA, Ubc13–Uev1A, Ub, and TAK1–TAB1–TAB2 complex (TRIKA2) as indicated. The reconstitution reaction mixture also contained ATP, E1, and 35S-labeled IκBα.