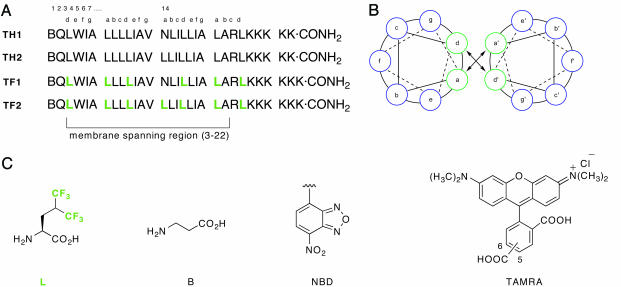
Fig. 2.
Peptide sequences, helical wheel diagram, and chemical structures of amino acids and fluorophore labels. (A) Sequences of the peptides used to demonstrate the ability of fluorinated amino acids to promote self-assembly. TH1 and TH2 contain leucine at the a and d positions of the putative micelle-embedded heptad repeats, whereas TF1 and TF2 contain hexafluoroleucine at these positions. (B) Helical wheel diagram depicting the membrane exposed residues (in blue) and the packing interface (green) in a parallel dimer. (C) Structures of the nonproteinogenic amino acids. Peptides labeled with fluorescence donor (NBD) and acceptor (TAMRA) at the N termini were used to assess oligomeric preferences by using FRET. A, Ala; I, Ile; K, Lys; L, Leu; N, Asn; Q, Gln; R, Arg; V, Val; and W, Trp.