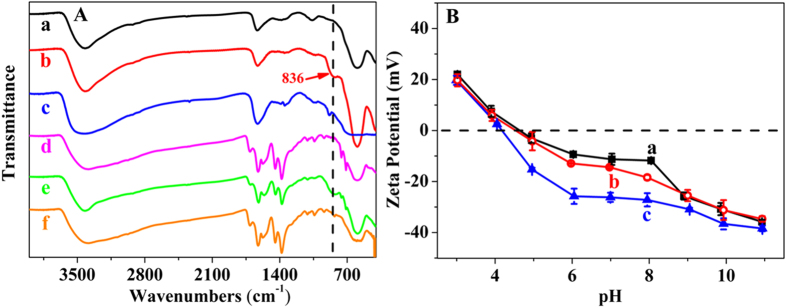
Figure 6. Adsorption mechanism study of iAs on the hybrid absorbent.
(A) FTIR spectra of (a) CoFe2O4, CoFe2O4 after (b) As(V) and (c) As(III) adsorption, (d) CoFe2O4@MIL-100(Fe), CoFe2O4@MIL-100(Fe) after (e) As(V) and (f) As(III) adsorption (10 mL of 100 mg L−1 iAs treated with 5 mg of the adsorbent). The peak at approximately 836 cm−1 was assigned to the stretching vibration of the Fe-O-As group. (B) Zeta potentials of (a) CoFe2O4@MIL-100(Fe) and CoFe2O4@MIL-100(Fe) after (b) As(III) and (c) As(V) adsorption (10 mL of 100 mg L−1 iAs treated with 5 mg of adsorbents). The zero charge point of the hybrid adsorbent was 4.7. The zeta potentials of CoFe2O4@MIL-100(Fe) decreased after iAs adsorption, suggesting the formation of negatively charged inner-sphere complexes between iAs and the hybrid adsorbent.