Abstract
The Havers‐Halberg Oscillation (HHO) hypothesis links evidence for the timing of a biorhythm retained in permanent tooth enamel (Retzius periodicity) to adult body mass and life history traits across mammals. Potentially, these links provide a way to access life history of fossil species from teeth. Recently we assessed intra‐specific predictions of the HHO on human children. We reported Retzius periodicity (RP) corresponded with enamel thickness, and cusp formation time, when calculated from isolated deciduous teeth. We proposed the biorhythm might not remain constant within an individual. Here, we test our findings. RP is compared between deciduous second and permanent first molars within the maxillae of four human children. Following this, we report the first RPs for deciduous teeth from modern great apes (n = 4), and compare these with new data for permanent teeth (n = 18) from these species, as well as with previously published values. We also explore RP in teeth that retain hypoplastic defects. Results show RP changed within the maxilla of each child, from thinner to thicker enameled molars, and from one side of a hypoplastic defect to the other. When considered alongside correlations between RP and cusp formation time, these observations provide further evidence that RP is associated with enamel growth processes and does not always remain constant within an individual. RP of 5 days for great ape deciduous teeth lay below the lowermost range of those from permanent teeth of modern orangutan and gorilla, and within the lowermost range of RPs from chimpanzee permanent teeth. Our data suggest associations between RP and enamel growth processes of humans might extend to great apes. These findings provide a new framework from which to develop the HHO hypothesis, which can incorporate enamel growth along with other physiological systems. Applications of the HHO to fossil teeth should avoid transferring RP between deciduous and permanent enamel, or including hypoplastic teeth.
Keywords: biorhythms, enamel growth, life history, Retzius lines
Introduction
Primate tooth enamel grows incrementally (Boyde, 1979, 1989). Each increment is marked by a growth line, as in shells and trees. One type of marking is Retzius lines (Retzius, 1837), which emerge on the outer lateral enamel surface as perikymata (e.g. Goodman & Rose, 1990). Retzius periodicity (RP) is the number of days of enamel growth between adjacent lines. The Havers‐Halberg Oscillation (HHO) hypothesis proposes that RP of permanent teeth is a manifestation of an underlying biorhythm that regulates growth, is associated with adult body mass, and is related to life history traits when compared between mammalian species (Bromage et al. 2009, 2012). The underlying cause of the biorhythm is unknown, though experimental research on domesticated pigs implicates resting metabolic rate as an important influence (Bromage et al. 2016).
This study builds upon our recent work in which we tested intra‐specific predictions of the HHO on human children (Mahoney et al. 2016). We reported that the modal and range of RPs from human deciduous teeth were lower than those calculated for human permanent teeth. Based upon this comparison, we suggested that RP might not remain constant within humans, though we did not calculate the periodicity of Retzius lines for deciduous and permanent teeth from the same individuals. We also reported that RP correlated with the reconstructed activity of enamel forming cells (secretory ameloblasts). The total amount of enamel deposited, and the time required by ameloblasts to form a human deciduous second maxillary molar cusp (dm2), were both correlated with RP. Correlation between RP and enamel formation time has been noted previously, within a sample of human permanent canines (Reid & Ferrell, 2006), and during inter‐specific comparisons of permanent first molars (M1) from extant and fossil hominoids (Mahoney et al. 2007). These correlations led us to suspect that RP might be related to some enamel growth processes.
The present study further investigates the possible links between RP and enamel growth. First, we compare RP between human deciduous and permanent molars within the maxillae of four human children. If the hypothesis that RP changes between these tooth types, from thinner to thicker enamel, is correct (Mahoney et al. 2016), then the timing of this growth rhythm should not remain constant within each maxilla. A deciduous molar from a fifth maxilla retained evidence of disturbed enamel growth in the form of a hypoplastic defect (see below). Relationships between non‐specific pathology and RP have not been examined previously. Yet, if, as we suspect, RP is linked to enamel growth, then perhaps disturbed enamel growth will be associated with RP in a deciduous crown.
In the second stage of this study we compare the timing of Retzius lines between deciduous and permanent teeth of great apes. We report the first deciduous RP values (n = 4) for modern orangutan (Pongo pygmaeus), gorilla (Gorilla gorilla), and chimpanzee (Pan troglodytes). These values are compared with new data for permanent teeth (n = 18) from these species, as well as with previously published values. Even though the deciduous and permanent teeth are not from the same individuals, we can still determine whether deciduous RPs are encompassed within the range of RPs for permanent teeth from each species. The present study will also contribute to a new baseline comparative dataset for great ape deciduous teeth. Retzius periodicity of permanent teeth is often compared between fossil and modern hominoids to gain insights into the evolution of dental development (e.g. Beynon et al. 1998; Schwartz et al. 2003; Mahoney et al. 2007), but rarely do such analyses include RP of deciduous teeth.
The timing of Retzius lines in humans and great apes
Retzius periodicity of modern human permanent teeth lies between a lowermost value of 6 days and an uppermost value of 12 days, with modes between 7 and 9 days depending upon the sample (Schwartz et al. 2001; Reid & Dean, 2006; Reid & Ferrell, 2006; Mahoney, 2008). The periodicity of 34 human deciduous teeth ranged between 4 and 11 days with a mode of 6 days (Mahoney et al. 2016). The lowered modal and range of RP values in this sample of isolated deciduous teeth, compared with permanent teeth, suggests the timing of Retzius lines might not remain constant within humans. However, one study reported that RP of a deciduous molar was the same as that observed in a permanent molar from the same mandible (Mahoney, 2012). Thus, it is still unclear whether RP changes between these tooth types in modern humans.
Modern orangutan permanent teeth have a range of RPs between 8 and 11 days, with a mode of 9 or 10 days (Beynon et al. 1991a; Dean, 2000; Schwartz et al. 2001; Kelley & Schwartz, 2010; Smith, 2016). Among modern gorillas, RP lies between 7 and 10 days, with a mode of 8 (females) and 9 days (males) (Beynon et al. 1991a; Schwartz et al. 2001; Kelley & Schwartz, 2010). The RP of modern chimpanzee permanent teeth might be as low as 5 days (Smith et al. 2010), but the majority of values range between 6 and 9 days (Reid et al. 1998; Schwartz et al. 2001), with a mode of 6 or 7 days (Schwartz et al. 2001; Smith et al. 2007). No study has reported the RP of great ape deciduous teeth.
Enamel hypoplastic defects
Disruptions to ameloblast activity during the secretory phase of enamel development can lead to hypoplastic defects that are retained in a tooth crown (Zsigmondy, 1893; Kreshover, 1940; Guatelli‐Steinberg, 2001 for a review). Hypoplastic defects, which are classified by their morphology as furrow, pit or plane‐type, can be visible from the external surface depending upon the angle that Retzius lines emerge in outermost enamel (Hillson & Bond, 1997; Guatelli‐Steinberg et al. 2012). These defects correspond with a range of non‐specific stressors in humans, including nutritional deficiencies (vitamin D and calcium), infectious diseases, fevers, and congenital syphilis (Sarnat & Schour, 1941; Sweeney et al. 1971; Purvis et al. 1973; Norén et al. 1978; Nikiforuk & Fraser, 1981; Goodman et al. 1987; May et al. 1993; Hillson et al. 1998; Berdal et al. 2005; Bossù et al. 2007). Unlike a localised hypoplasia (Goodman & Rose, 1990), these systemic events can disrupt enamel growth in all forming crowns at the same time.
Hypoplastic enamel can be less mineralised, softer, and contain smaller hydroxyapatite crystallites, relative to normal enamel (Suckling et al. 1989; Batina et al. 2004). An altered microstructure implies that ameloblasts did not recover from the stress event that occurred during enamel secretion, and this affected subsequent maturation (Suckling et al. 1989; Batina et al. 2004). Hypoplastic enamel can also be as hard as normal enamel, indicating that maturation resumed after the defective secretory phase (Suckling & Purdell‐Lewis, 1982; Suckling et al. 1989). Thus, disruptions to ameloblast activity can either be temporary or more sustained, which might relate in part to the stage of cell activity (Suga, 1989).
Materials and methods
Five human juvenile skeletons with erupted dm2 (n = 5) and erupting maxillary M1s (n = 4) were selected (Table 1). The skeletons dated to the mediaeval period (11th to 15th century AD) in England (Hicks & Hicks, 2001) and are curated in the Skeletal Biology Research Centre, University of Kent, UK. The accession numbers are NGB 1988, Sk27; NGA 1989, Sk102, 178, 665, 671. One dm2 retained evidence of a hypoplastic defect, which was systemic, as we observed a corresponding defect in cervical enamel of dm1 from the same maxilla.
Table 1.
Retzius periodicity in humans
| Sk | RP in days | |
|---|---|---|
| Udm2 | UM1 | |
| 27a | 4–5 | |
| 102 | 6 | 7 |
| 178 | 9 | 10 |
| 665 | 7 | 10 |
| 671 | 10 | 8 |
Sk, Skeletal number.
Tooth types: Udm2, upper second deciduous molar; UM1, upper first permanent molar.
Hypoplastic.
Thin sections of four deciduous teeth from great apes were chosen for this study. One deciduous second mandibular molar (dm2) from P. pygmaeus and G. gorilla, and one deciduous mandibular canine (dc1) from P. troglodytes were selected from the Elliot Smith Collection, housed in the Anatomy Lab, University College London, UK. These sections were selected because it was possible to reconstruct RP accurately. The apes were wild shot specimens from the 1920s. Thin sections from these specimens were first prepared for a paper on tooth wear by Aiello et al. (1991). The accession numbers are (Orangutan) J56‐E, (Gorilla) CA1F‐1472‐E, and (Pan) CA20A‐2‐36. Another dc1 from P. troglodytes (906‐11‐73) was selected from a collection of primate sections held at The Ohio State University.
Thin sections of 18 ape permanent teeth were selected from the Elliot Smith Collection. These were a mix of maxillary and mandibular permanent first, second, and third molars of P. troglodytes (n = 8: accession numbers CA‐11, CA‐13D, CA‐14, CA‐14A two slides, CA‐14E, CA‐19B, D‐Case), permanent premolars and molars of G. gorilla (n = 6: accession numbers HT41‐89 two slides, HT42‐89, HT44‐89, UCL‐CA‐18, UCL‐CA‐4), and permanent premolars and molars of P. pygmaeus (n = 4: accession numbers HT‐162/88 two slides, HT‐166/88, HT‐1/91). No permits were needed to examine the deciduous or permanent slides.
Sample preparation
The human molars were prepared using standard methods (e.g. Mahoney, 2008). Each tooth was embedded in polyester resin to reduce the risk of splintering while sectioning. Using a diamond‐wafering blade (Buehler® IsoMet 4000 precision saw), sections were taken through the outermost enamel cusp tip, the tip of the dentine horn, and the most cervical enamel extension. Each section was mounted on a microscope slide, and lapped (Buehler® Eco‐Met 300) using a graded series of grinding pads (ranging in grit size from P400 to P1200) to reveal incremental lines. Each section was polished with an aluminium oxide powder (Buehler® Micro‐Polish II: 0.3 μm) placed in an ultrasonic bath to remove surface debris, dehydrated through alcohol baths, cleared (Histoclear®), and mounted with a coverslip using a xylene‐based mounting medium (DPX®).
Microscopy
All sections were examined at magnification (20–60×) using a high‐resolution microscope (Olympus® BX51). Images were captured with a microscope digital camera (Olympus® DP25) and analysed in CELL® Live Biology imaging software. RPs for human juveniles were recorded over a 5‐year period. Each slide was recorded four times. If values were not the same from one recording to the next, then the slide was not included in this study.
We calculated RP in post‐natal lateral enamel, avoiding cervical enamel immediately adjacent to the tooth cervix, because the ‘packing’ effect of Retzius lines in this region makes it difficult to calculate their periodicity. In humans, dm2 lateral enamel forms from about 3 months after birth, to around the end of the first post‐natal year (see Mahoney, 2015 for data; and discussion in Mahoney et al. 2016). A neonatal line, the marker between pre‐, and post‐natal enamel, was located in cuspal enamel of the great ape dm2s (which can be seen in the corresponding Figure of the orangutan dm2 reported in the Results section). Cuspal enamel forms before lateral enamel. The word ‘cuspal’ refers to enamel that forms over the dentine horn, excluding lateral and cervical enamel. The word ‘cusp’ (e.g. protocone, or metacone cusp) refers to the first formed enamel over the dentine horn to the last formed enamel at the cervix.
A neonatal line, with a corresponding accentuated marking in dentin, was located towards the end of cuspal enamel growth in the chimpanzee dc1 from the UCL collection. RP was calculated for this dc1 from Retzius lines that were present in lateral enamel, just after the neonatal line. A neonatal line was not present in the chimpanzee dc1 from The Ohio State University collection. We recorded Retzius lines in the most apical lateral enamel of this tooth.
The number of daily enamel growth increments (cross‐striations) was counted along a rod between two adjacent Retzius lines of one human molar, the orangutan deciduous molar, and two ape permanent molars. Cross‐striations correspond with a circadian rhythm (Lacruz et al. 2012; Zheng et al. 2013). For all other sections, RP was calculated by measuring the distance between Retzius lines of lateral enamel. The measurement was divided by average local daily enamel secretion rates (DSRs; Mahoney, 2008 for a methodology).
Average enamel thickness
Average enamel thickness (AET) was calculated by dividing the area of the enamel crown by the length of the dentine‐enamel junction (DEJ), which provides the average straight‐line distance in mm between the DEJ and outer enamel surface (Martin, 1983, 1985).
Results
RP in human deciduous and permanent enamel
Human RP data are in Table 1. In the maxillae of three children, RP increased from dm2 with a lower mean AET of 0.69 mm to M1 with a higher mean AET of 1.01 mm. In one maxilla, RP decreased from 10 days in a dm2 with an AET of 0.89 mm to 8 days when compared with M1 with an AET of 0.81 mm.
RP in a crown with hypoplasia
Figure 1 illustrates the enamel defect and Retzius lines. The average distance of 14.5 μm between two adjacent lines in mesio‐buccal cusp lateral enamel, before the defect formed, divided by a local average DSR of 3.81 μm, gave an RP of 4 days. When the analysis was repeated on an equivalent region of the mesio‐lingual cusp, it gave an RP of 4 days. The average distance of 21 μm between two Retzius lines in cervical enamel, after the defect formed, divided by a local average DSR of 4.10 μm, gave an RP of 5 days.
Figure 1.
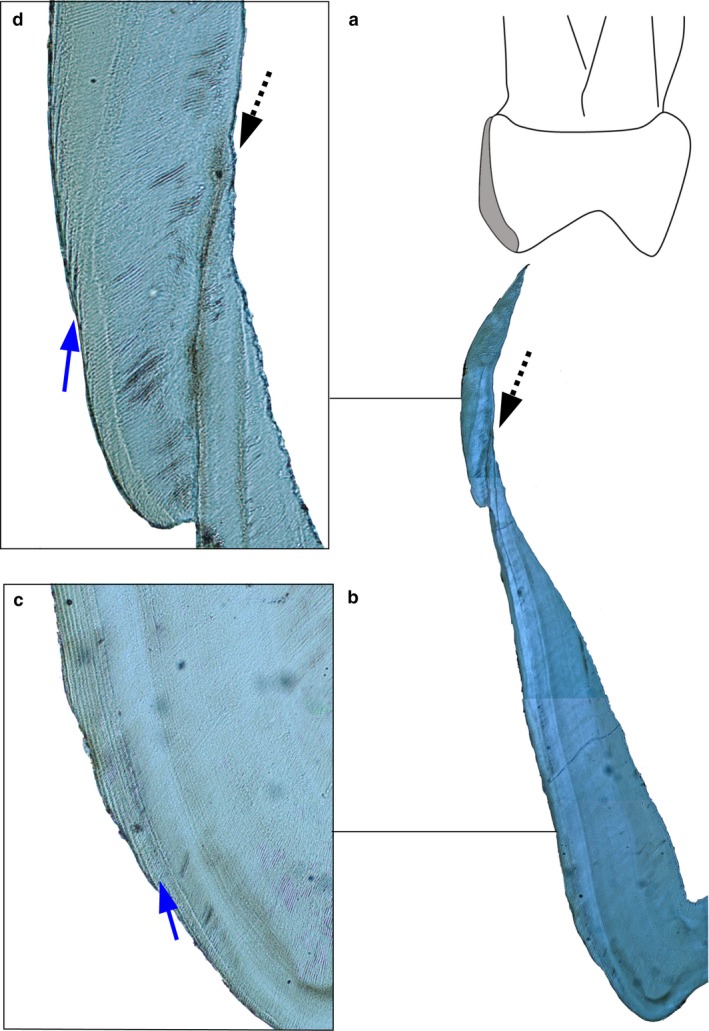
Hypoplastic defect and Retzius periodicity (zoom in to see Retzius lines). (a) Human deciduous maxillary second molar mesio‐lingual enamel highlighted in grey. (b) The same region imaged using a polarizing lens. Dashed arrow points to a hypoplastic defect associated with an accentuated marking. Magnification = 4×. (c) Blue arrow points to Retzius lines that formed before the hypoplastic defect. Magnification = 20×. (d) Blue arrow points to Retzius lines that formed after the hypoplastic defect. The stress event did not prevent secretory ameloblasts from recovering, as these cells had a functional Tomes process (separate rods are visible) that deposit enamel at a slightly accelerated rate.
RP in great ape deciduous and permanent enamel
Retzius periodicity data for great apes are presented in Table 2. Figure 2 illustrates a direct count of cross‐striations between adjacent Retzius lines in the mesio‐lingual cusp of the orangutan dm2, which was 5 days. Periodicity for the mesio‐lingual cusp of the gorilla dm2 was 5 days. When the analysis was repeated on the mesio‐buccal cusp of the gorilla molar, it gave a count of 5 days. RP of the chimpanzee dc1 from the UCL collection was 5 days. The periodicity of the dc1 from the Ohio collection was either 5 or 6 days.
Table 2.
Retzius periodicity in great apes
| Species | RP in days | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Deciduous | ||||||||
| Pan troglodytes | 1 | 1a | ||||||
| Gorilla gorilla | 1 | |||||||
| Pongo pygmaeus | 1 | |||||||
| Permanent | ||||||||
| Pan troglodytes | 1 | 4 | 2 | 1 | ||||
| Gorilla gorilla | 5 | 1 | ||||||
| Pongo pygmaeus | 3 | 1b | ||||||
The RP of this lower deciduous canine was either 5 or 6 days.
The RP calculated in the lateral enamel of the mesio‐buccal cusp of this premolar was 12 days. When the analysis was repeated in the mesio‐lingual cusp lateral enamel of the premolar it gave an RP of 12 days.
Figure 2.
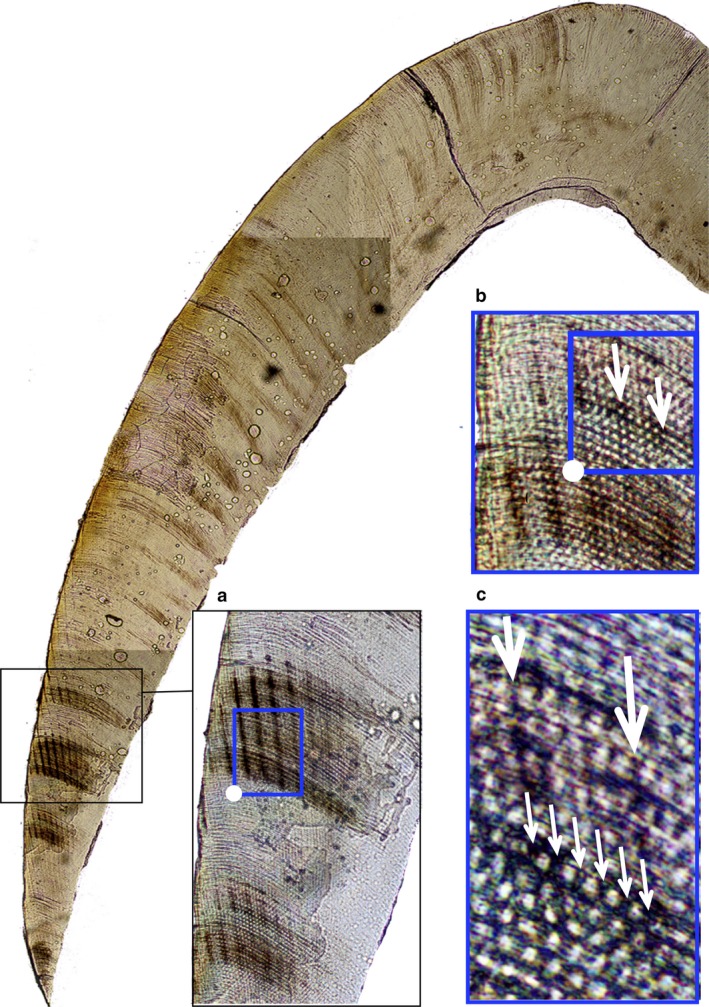
Retzius periodicity in a juvenile orangutan lower second deciduous molar. (a) Retzius lines in cervical enamel. Magnification = 4×. (b) Daily cross‐striations. White arrows point to the first and last cross‐striation between two adjacent Retzius lines (zoom in to see). Magnification = 20×. (c) Large white arrows point to the same two adjacent Retzius lines. Smaller white arrows point to cross‐striations, corresponding to 5 days of enamel secretion. Magnification = 60×.
Retzius periodicity of permanent teeth was between 10–12 days for P. pygmaeus, 7–8 days for G. gorilla, and 5–8 days for P. troglodytes. The one uppermost value of 12 days for P. pygmaeus extends the known range of RPs from permanent teeth for this species by 1 day.
Discussion
The present study builds upon our previous work by showing that in humans, within the same individual, RP can change from deciduous to permanent teeth. Our data also suggest that this may be the case in great apes, although RP differences between deciduous and permanent teeth of the same individuals would be necessary to confirm this hypothesis. Our study further suggests that RP can change on either side of a hypoplastic defect, where both a higher RP and an increase in daily secretion rates can occur after the defect has formed. Combined, these observations indicate that if RP is a systemic rhythm governed by supra‐chiasmic nuclei (in the hypothalamus), then it appears that it does not always remain constant over an individual's lifespan, as previously assumed (Bromage et al. 2009). Instead, the timing of Retzius lines within an individual will either remain constant (Mahoney, 2012), or may vary by up to 3 days, from deciduous to permanent teeth (Tables 1 and 3).
Table 3.
Retzius periodicity and daily secretion rates in hypoplastic teeth
| Enamel Region | LI2 | LC1 | LC1 | |||
|---|---|---|---|---|---|---|
| RP, days | DSR, μm | RP, days | DSR, μm | RP, days | DSR, μm | |
| Before defect | 8 | 4.43 | 6 | 3.57 | 11 | 4.25 |
| During defect | 8 | 3.51 | 6 | 3.36 | 11 | 3.57 |
| After defect | 10 | 3.93 | 8 | 3.99 | 11 | 4.02 |
DSR, mean daily enamel secretion rates in outer enamel.
Tooth types: LI2, lower lateral permanent incisor; LC1, lower permanent canine.
RP in human deciduous and permanent enamel
An increase in RP from deciduous to permanent molars from the same individual is consistent with our previous finding that the timing of Retzius lines is associated with enamel thickness (Mahoney et al. 2016). However, in one maxilla where RP decreased from a deciduous to a permanent molar, the dm2 was slightly larger, with thicker enamel, compared with M1. Normally, dm2 has an AET that is less than M1. Sometimes, though, permanent first molars can be slightly smaller than their deciduous precursors (Moorrees & Reed, 1964), and their range of AET values can overlap (dm2 range = 0.42–1.04; M1 range = 0.82–1.21; Skinner et al. 2015; Mahoney et al. 2016). These data suggest that RP can change with age for human children when enamel is thicker, or thinner, in later‐forming permanent molars, relative to deciduous molars.
Several factors contribute to primate tooth enamel thickness. One is RP, as we have shown. The number of active ameloblasts, their secretory life span and the time taken to form regions of a crown, as well as the rate these cells secrete enamel matrix, also relate to enamel thickness (Grine & Martin, 1988; Macho, 1995; Dean, 2000; Dean et al. 2001; Mahoney, 2011). It is not surprising therefore that RP correlates with the time required to form dm2 paracone cusp enamel (Mahoney et al. 2016). Shorter total crown formation times, thinner enamel, and lowered RPs of deciduous compared with permanent teeth (Reid & Dean, 2006; Mahoney, 2011, 2012; Mahoney et al. 2016) are consistent with this idea. RP also correlates with permanent canine lateral enamel formation time (Reid & Ferrell, 2006), though this might relate to the duration of enamel extension. Whether there is also an association between the length of the enamel‐dentine junction and enamel thickness of permanent canines, when for example smaller teeth are compared with larger teeth, has yet to be determined.
Two additional analyses were undertaken to further explore RP and the amount, and rate, of enamel deposition within enamel ‘layers’. The distance between two adjacent Retzius lines in 14 human dm2s from different individuals, was compared with RP from the same teeth (Fig. 3). RPs were observed and measured in homologous locations, in outer lateral post‐natal enamel, within each of the crowns. The distance between lines was significantly and positively correlated with RP (Pearson's r = 0.940, P < 0.000). Thus, higher RPs are associated with thicker enamel ‘layers’ in this sample of teeth because there are a greater number of days – more cross‐striations – between each ‘beat’ of the biorhythm. However, thicker enamel ‘layers’ between Retzius lines of higher periodicity were not accompanied by a clear change in the rate that ameloblasts secrete enamel. Mean DSRs in mid to outer lateral enamel of molars with RPs of 4–7 days ranged between 3.44 and 4.20 μm (one outlier of 5.10 μm), overlapping with mean DSRs of 3.50–4.50 μm from molars with RPs of 9– 11 days. These data suggest that if the rate that enamel matrix is deposited between adjacent Retzius lines varies only slightly, higher RPs, combined with ameloblasts that have longer secretory life spans, should lead to thicker enamel on molar crowns. Our results imply that, when secretion rates are constrained, RP variation appears to be a major contributor to enamel thickness, when equivalent enamel regions from one tooth type are compared between individuals.
Figure 3.
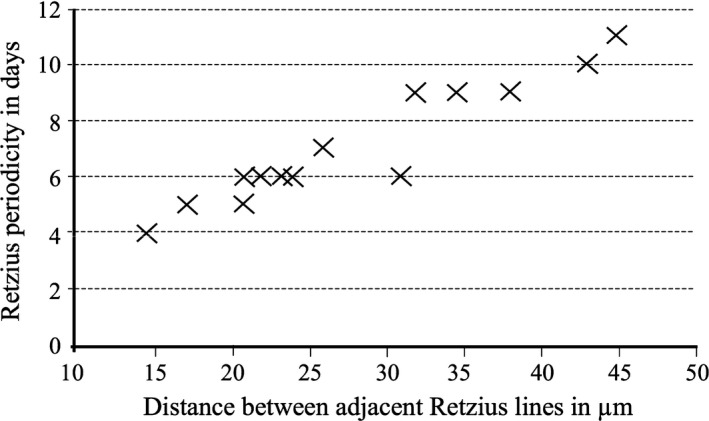
Scatter plot of dm2 Retzius periodicity against Retzius line spacing. There is a significant (P < 0.000) and positive correlation between the two variables.
Retzius periodicity was calculated high in outer lateral enamel and compared with RP low in outer cervical enamel of the same section, for three permanent second molars. Retzius periodicity did not change between these locations in each molar (RP of 7, 8, and 10 in each molar, respectively). This makes sense, because here – unlike the comparison of RP between 14 dm2s above – secretion rates are not constrained, as they vary greatly from one enamel region to the next in human permanent molars (e.g. Lacruz & Bromage, 2006; their Table 2). In the three molars examined here, DSRs ranged between 4.65 and 5.09 μm high in outer lateral enamel, and between 2.58 and 3.10 μm low in outer cervical enamel. The spacing of Retzius lines, as well as their surface manifestation as perikymata, also become compressed in cervical compared with lateral enamel (e.g. Beynon et al. 1991b; Dean & Reid, 2001; Reid & Ferrell, 2006; Guatelli‐Steinberg et al. 2007). Thus, the narrow enamel layers that form towards the end of a crown's growth period, do so slowly, leading to the same RP as the thicker enamel layers of lateral regions, which form relatively faster and earlier on in crown growth. In each of these enamel regions, the number of cross‐striations between adjacent Retzius lines remains constant, even though the amount of enamel deposited, and the spacing between the lines, changes. Thus, the relationship between RP and enamel layers is much weaker when DSRs are more variable. Our results imply that the timing of Retzius lines does not vary within a ‘healthy’ molar crown.
Factors that contribute to enamel thickness are not constant from one tooth type to the next when compared along the row (e.g. Mahoney, 2015). Given that RP can be associated with enamel thickness, there is reason to suspect that these associations will also not transfer unchanged from one tooth type to the next, in any one individual. That is, relationships between RP, and enamel growth and thickness, are likely to be relative within a tooth type. For example, large portions of enamel forming at the same time in different deciduous teeth, such as maxillary lateral incisors and first molars, might have equivalent RPs that are associated with very different developmental pathways. Ameloblasts secrete enamel at an accelerated rate in deciduous incisors but have a shortened secretory life span, leading to a thinner enamel crown, compared with molars (Mahoney, 2010, 2011, 2012, 2013). Theoretically, accelerated ameloblast secretion rates of incisors could produce thickened enamel layers, relative to enamel layering in molars with the same RPs that have slower secretion rates. Thicker enamel layers of deciduous incisors would then be associated with a thinner incisor enamel crown compared with deciduous molars (see also section on Developing the HHO below).
RP and hypoplasia
A change in the timing of Retzius lines, from one side of a hypoplastic defect to the other, in a deciduous crown suggests that RP can be modulated by local systemic stress events. A period of ‘catch up growth’ in enamel secretion, after a period of reduced secretion, has been documented previously (e.g. Macchiarelli et al. 2006; Mahoney, 2008), but an increase in RP after a hypoplastic lesion is a new observation. We observed greater spacing between Retzius lines in cervical enamel after a hypoplastic defect, which also has been reported for enamel of domestic pig and wild boar (Witzel et al. 2006, 2008 see their fig. 8a). Slightly accelerated average DSRs in cervical compared with lateral enamel were also unexpected because, like permanent teeth, rates usually decrease towards the end of the growth period in deciduous crowns (Mahoney, 2011). The greater distance between Retzius lines and accelerated secretion rates suggest that ameloblasts deposited more enamel between each ‘beat’ of the underlying biorhythm, after recovering from a stress event that led to a hypoplastic defect.
One futher analysis was undertaken to explore RP in three isolated permanent teeth that retained evidence of hypoplastic defects (Table 3). In two of these teeth, RP changed, increasing from one side of the defect to the other. Like the hypoplastic deciduous tooth, secretion rates also accelerated after the defect formed in two permanent teeth, and this was combined with a slower beat of the biorhythm, leading to a higher RP and an increased spacing between Retzius lines. These preliminary data from a few teeth imply that ameloblast secretion rates and the underlying biorhythm can both respond to systemic non‐specific pathology.
RP in great ape deciduous and permanent enamel
Retzius periodicity of deciduous teeth from P. pygmaeus and G. gorilla extends below the lowermost RPs we observed in permanent molars from these species (Table 2), as well as those reported previously (Schwartz et al. 2001; Kelley & Schwartz, 2010). RPs of two deciduous canines from P. troglodytes lie within the lower range of RPs from permanent teeth (see our Table 2; Schwartz et al. 2001; Smith et al. 2010). Clearly, the extent of similarities or differences in RP of deciduous and permanent enamel from great apes has yet to be determined. Nevertheless, the deciduous RPs are all low compared with RPs from permanent teeth of great apes.
Lower RPs from ape deciduous teeth are consistent with the proposal that RP may be linked to enamel thickness, and at least one underlying enamel growth mechanism – formation time. The orangutan dm2 AET of 0.53 mm (0.4–0.5 mm: Zanolli et al. 2015) extends below the lowermost AET of 0.77 mm from permanent molars of this species (Skinner et al. 2015). Further analysis of the dm2 reveals a mesio‐buccal cusp formation time of 396 days (see Mahoney, 2011 for method), which lies outside the lowermost formation time of 1006 days reported from an analysis of six permanent M1 mesio‐buccal cusps of P. pygmaeus (Smith, 2016). The gorilla dm2 AET of 0.54 mm extends below the lowermost AET of 0.79 mm for permanent molars (Skinner et al. 2015). Further analysis of the dm2 reveals a mesio‐buccal cusp formation time of 366 days (see Mahoney, 2011 for method), which is less than the formation time of 843–891 days reported for two permanent M1 mesio‐buccal cusps of G. gorilla. No study has reported AET for permanent maxillary canines from Pan.
Developing the HHO
More work is needed to understand the interaction between the different factors that contribute to enamel thickness, and the timing of Retzius lines. Perhaps crown extension in height combined with enamel thickness and crown formation time will show some associations with RP, given the stretching of the ameloblast sheet that has been demonstrated and modelled previously (Shellis, 1984). Disentangling these relationships will benefit the development of the HHO. For example, lower RPs were associated with longer lateral enamel formation times within a sample of permanent canines, whereas higher RPs were related to longer cusp formation times within a sample of deciduous molars (Reid & Ferrell, 2006; Mahoney et al. 2016).
Enamel thickness increases along the human tooth row, from first to third permanent molars (Grine, 2005). We have shown that RP is a major contributor to enamel thickness when DSR variation is constrained. Whether RP is associated with enamel thickness when compared between analogous regions along the molar row from the same individuals has yet to be determined. Future studies might incorporate an assessment of RP, enamel thickness, DSRs and the length of time over which ameloblasts secrete enamel. One such approach would be to count the total number of cross‐striations along an enamel prism, calculate DSRs along the prism length, and then assess how those numbers correspond with RP. Based upon our findings, it would seem likely that all three variables – RP, DSR, and the length of time over which ameloblasts secrete enamel – need to be considered and incorporated into predictions of how these factors affect enamel thickness.
Future studies might explore associations we have reported across primates. For example, AET of human permanent molars ranges between 0.67 and 2.30 mm (Olejniczak et al. 2008), which coincides with a range of RPs of between 6 and 12 days. AET of Pan molars ranges between 0.58 and 0.94 mm (Skinner et al. 2015), which coincides with an RP of 5–9 days. If RP is linked to permanent enamel thickness and/or underlying enamel formation processes, then these different ranges might be expected. Given that enamel thickness relates to lifespan and high‐wear diets across primates (Pampush et al. 2013), such analyses may potentially reveal new ways to explore the timing of life history traits.
Conclusion
Our data have shown that RP can change within human children. Preliminary insights suggest great ape dentition might follow a similar pattern. When these data are considered alongside altered RPs within a crown, from one side of a hypoplastic disruption to the other, as well as correspondence between RP and the amount of enamel deposited within an ‘enamel layer’, it suggests that the timing of Retzius lines is linked to enamel growth. If RP is a measure of an underlying systemic biorhythm that affects multiple physiological systems (Bromage et al. 2012), then we conclude that the influence of the biorhythm extends to enamel growth, can be modulated by local stress events, and may even be expressed differently in enamel of different thickness and/or in teeth with contrasting secretion rates and formation times.
Acknowledgements
We thank the Editor and two anonymous reviewers for comments that improved the manuscript.
References
- Aiello LC, Dean MC, Montgomery C (1991) The natural history of deciduous tooth attrition in hominoids. J Hum Evol 21, 397–412. [Google Scholar]
- Batina N, Renugopalakrishnan V, Casillas Lavin PN, et al. (2004) Ultrastructure of dental enamel afflicted with hypoplasia: an a atomic force microscopic study. Calcif Tissue Int 74, 294–301. [DOI] [PubMed] [Google Scholar]
- Berdal A, Bailleul‐Forestier I, Davideau J‐L, et al. (2005) Dento‐alveolar bone complex and vitamin D In: Vitamin D (eds Feldman D, Pike JW, Glorieux FH.), pp. 599–607. San Diego: Elsevier Academic Press. [Google Scholar]
- Beynon AD, Dean MC, Reid DJ (1991a) Histological study on the chronology of the developing dentition in Gorilla and Orangutan. Am J Phys Anth 86, 189–203. [Google Scholar]
- Beynon AD, Dean MC, Reid DJ (1991b) On thick and thin enamel in hominoids. Am J Phys Athropol 86, 295–309. [Google Scholar]
- Beynon AD, Dean MC, Leakey MG, et al. (1998) Comparative dental development and microstructure of Proconsul teeth from Rusinga Island, Kenya. J Hum Evo 35, 163–209. [DOI] [PubMed] [Google Scholar]
- Bossù M, Bartoli A, Orsini G, et al. (2007) Enamel hypoplasia in coeliac children: a potential clinical marker of early diagnosis. Eur J Paediatr Dent 8, 31–37. [PubMed] [Google Scholar]
- Boyde A (1979) Carbonate concentration, crystal centres, core dissolution, caries, cross striation, circadian rhythms and compositional contrast in the SEM. J Dent Res 58, 981–983. [DOI] [PubMed] [Google Scholar]
- Boyde A (1989) Enamel In: Teeth. Handbook of Microscopic Anatomy (eds Berkovitz BKB, Boyde A, Frank RM, et al.), pp 309–473. Berlin: Springer‐Verlag. [Google Scholar]
- Bromage TG, Lacruz RS, Hogg R, et al. (2009) Lamellar bone is an incremental tissue reconciling enamel rhythms, body size, and organismal life history. Calcif Tissue Int 84, 388–404. [DOI] [PubMed] [Google Scholar]
- Bromage TG, Hogg RT, Lacruz RS, et al. (2012) Primate enamel evinces long period biological timing and regulation of life history. J Theor Biol 305, 131–144. [DOI] [PubMed] [Google Scholar]
- Bromage TG, Idaghdour Y, Lacruz RS, et al. (2016) The swine plasma metabolome chronicles ‘many days’ biological timing and functions linked to growth. PLoS One 11, e0145919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean MC (2000) Progress in understanding hominoid dental development. J Anat 197, 77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean MC, Reid DJ (2001) Perikymata spacing and distribution on hominid anterior teeth. Am J Phys Anthropol 116, 209–215. [DOI] [PubMed] [Google Scholar]
- Dean MC, Leakey MG, Reid DJ, et al. (2001) Growth processes in teeth distinguish modern humans from Homo erectus and earlier hominins. Nature 414, 628–631. [DOI] [PubMed] [Google Scholar]
- Goodman AH, Rose JC (1990) Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Ybk Phys Anthropol 33, 59–110. [Google Scholar]
- Goodman AH, Allen LH, Hernandez GP, et al. (1987) Prevalence and age at development of enamel hypoplasias in Mexican children. Am J Phys Anthropol 72, 7–19. [DOI] [PubMed] [Google Scholar]
- Grine FE (2005) Enamel thickness of deciduous and permanentmolars in modern Homo sapiens . Am J Phys Anthropol 126, 14–31. [DOI] [PubMed] [Google Scholar]
- Grine FE, Martin LB (1988) Enamel thickness and development in Australopithecus and Paranthropus In: The Evolutionary History of the Robust Australopithecines (ed. Grine FE.), pp. 3–42. New York: Aldyne de Gruiter. [Google Scholar]
- Guatelli‐Steinberg D (2001) What can developmental defects of enamel reveal about physiological stress in nonhuman primates? Evo Anth 10, 138–151. [Google Scholar]
- Guatelli‐Steinberg D, Reid DJ, Bishop T (2007) Did the lateral enamel of Neandertals grow differently from that of modern humans? J Hum Evol 52, 72–84. [DOI] [PubMed] [Google Scholar]
- Guatelli‐Steinberg D, Ferrell RJ, Spence J (2012) Linear enamel hypoplasia as an indicator of physiological stress in great apes: reviewing the evidence in light of enamel growth variation. Am J Phys Anthropol 148, 191–204. [DOI] [PubMed] [Google Scholar]
- Hicks M, Hicks A (2001) St. Gregory's Priory, Northgate, Canterbury Excavations 1988–1991. Canterbury Archaeological Trust Ltd: Volume II.
- Hillson S, Bond S (1997) The relationship of enamel hypoplasia to the pattern of tooth crown growth: a discussion. Am J Phys Anthropol 104, 89–103. [DOI] [PubMed] [Google Scholar]
- Hillson S, Grigson C, Bond S (1998) Dental defects of congenital syphilis. Am J Phys Anthropol 107, 25–40. [DOI] [PubMed] [Google Scholar]
- Kelley J, Schwartz GT (2010) Dental development and life history in living African and Asian apes. PNAS 107, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreshover SJ (1940) Histopathologic studies of abnormal enamel formation in human teeth. Am Orthodont Oral Surg 26, 1083–1101. [Google Scholar]
- Lacruz RS, Bromage TG (2006) Appositional enamel growth in molars of South African fossil hominids. J Anat 209, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Hacia JG, Bromage TG, et al. (2012) The circadian clock modulates enamel development. J Biol Rhyth 27, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarelli R, Bondioli L, Debénath A, et al. (2006) How Neanderthal molar teeth grew. Nature 444, 748–751. [DOI] [PubMed] [Google Scholar]
- Macho G (1995) The significance of hominid enamel thickness for phylogenetic and life‐history reconstruction In: Aspects of Dental Biology: Palaeontology, Anthropology and Evolution (ed. Moggi‐Cecchi J.), pp. 51–68. Florence: International Institute for the Study of Man. [Google Scholar]
- Mahoney P (2008) Intraspecific variation in M1 enamel development in modern humans: implications for human evolution. J Hum Evol 55, 131–147. [DOI] [PubMed] [Google Scholar]
- Mahoney P (2010) Two dimensional patterns of human enamel thickness on deciduous (dm1, dm2) and permanent first (M1) mandibular molars. Arch Oral Biol 55, 115–126. [DOI] [PubMed] [Google Scholar]
- Mahoney P (2011) Human deciduous mandibular molar incremental enamel development. Am J Phys Anthropol 144, 204–214. [DOI] [PubMed] [Google Scholar]
- Mahoney P (2012) Incremental enamel development in modern human deciduous anterior teeth. Am J Phys Anthropol 147, 637–651. [DOI] [PubMed] [Google Scholar]
- Mahoney P (2013) Testing functional and morphological interpretations of enamel thickness along the deciduous tooth row in human children. Am J Phys Anthropol 151, 518–525. [DOI] [PubMed] [Google Scholar]
- Mahoney P (2015) Dental fast track: prenatal enamel growth, incisor eruption, and weaning in human infants. Am J Phys Anthropol 156, 407–421. [DOI] [PubMed] [Google Scholar]
- Mahoney P, Smith T, Schwartz G, et al. (2007) Molar crown formation in the late Miocene Asian hominoids, Sivapithecus parvada and Sivapithecus sivalensis . J Hum Evol 53, 61–66. [DOI] [PubMed] [Google Scholar]
- Mahoney P, Miszkiewicz JJ, Pitfield R, et al. (2016) Biorhythms, deciduous enamel thickness, and primary bone growth in modern human children: a test of the Havers‐Halberg Oscillation hypothesis. J Anat 228, 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB (1983). Relationships of the later Miocene Hominoidea. Ph.D. Dissertation, University College London.
- Martin LB (1985) Significance of enamel thickness in hominoid evolution. Nature 314, 260–263. [DOI] [PubMed] [Google Scholar]
- May RL, Goodman AH, Meindl RS (1993) Response of bone and enamel formation to nutritional supplementation and morbidity among malnourished Guatemalan children. Am J Phys Anthropol 92, 37–51. [DOI] [PubMed] [Google Scholar]
- Moorrees CFA, Reed RB (1964) Correlations among crown diameters of human teeth. Arch Oral Biol 9, 685–697. [DOI] [PubMed] [Google Scholar]
- Nikiforuk G, Fraser D (1981) The etiology of enamel hypoplasia: a unifying concept. J Pediatr 98, 888–893. [DOI] [PubMed] [Google Scholar]
- Norén JG, Magnusson BO, Grahnén H (1978) Mineralisation defects of primary teeth in intra‐uterine undernutrition. A histological and microradiographic study. Swed Dent J 2, 67–72. [PubMed] [Google Scholar]
- Olejniczak AJ, Smith TM, Wang W, et al. (2008) Molar enamel thickness and dentine horn height in Gigantopithecus blacki . Am J Phys Anthropol 135, 85–91. [DOI] [PubMed] [Google Scholar]
- Pampush JD, Duque AC, Burrows BR, et al. (2013) Homoplasy and thick enamel in primates. J Hum Evol 64, 216–224. [DOI] [PubMed] [Google Scholar]
- Purvis RJ, Mackay GS, Cockburn F, et al. (1973) Enamel hypoplasia of the teeth associated with neonatal tetany: a manifestation of maternal vitamin‐D deficiency. Lancet 302, 811–814. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Dean MC (2006) Variation in modern human enamel formation times. J Hum Evol 50, 329–346. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Ferrell R (2006) The relationship between number of striae of Retzius and their periodicity in imbricational enamel formation. J Hum Evol 50, 195–202. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Schwartz GT, Dean MC, et al. (1998) A histological reconstruction of dental development in the common chimpanzee, Pan troglodytes . J Hum Evol 35, 427–448. [DOI] [PubMed] [Google Scholar]
- Retzius A (1837) Bemerkungen uber den innern Bau der Zahne, mit besonderer Rucksicht auf den im Zahnknochen vorkommenden Rohrenbau In: Archiv fur Anatomie, Physiologie und Wissenschaftliche Medicin, in Verbindung mit Gelehrten (ed. Muller J.), pp. 486–566. Berlin: Verlag von W. Thome. [Google Scholar]
- Sarnat BG, Schour I (1941) Enamel hypoplasias (chronologic enamel hypoplasia) in relation to systemic diseases: a chronological, morphological and etiological classification. J Am Dent Assoc 28, 1989–2000. [Google Scholar]
- Schwartz GT, Reid DJ, Dean C (2001) Developmental aspects of sexual dimorphism in hominoid canines. Int J Primatol 22, 837–860. [Google Scholar]
- Schwartz GT, Liu W, Zheng L (2003) Preliminary investigation of dental microstructure in the Yuanmou hominoid (Lufengpithecus hudienensis), Yunnan Province, China. J Hum Evol 44, 189–202. [DOI] [PubMed] [Google Scholar]
- Shellis RP (1984) Variations in growth of the enamel crown in human teeth and a possible relationship between growth and enamel structure. Arch Oral Biol 29, 671–682. [DOI] [PubMed] [Google Scholar]
- Skinner MM, Alemseged Z, Gaunitz C, et al. (2015) Enamel thickness trends in Plio‐Pleistocene hominin mandibular molars. J Hum Evol 85, 35–45. [DOI] [PubMed] [Google Scholar]
- Smith TM (2016) Dental development in living and fossil orang‐utans. J Hum Evol 94, 92–105. [DOI] [PubMed] [Google Scholar]
- Smith TM, Reid DJ, Dean MC, et al. (2007) New perspectives on chimpanzee molar crown development In: Dental Perspectives in Human Evolution: State of the Art Research in Dental Anthropology (eds Bailey S, Hublin JJ.), pp. 177–192. Berlin: Springer‐Verlag. [Google Scholar]
- Smith TM, Smith BH, Reid DJ, et al. (2010) Dental development of the Taï Forest chimpanzees revisited. J Hum Evol 58, 363–373. [DOI] [PubMed] [Google Scholar]
- Suckling GW, Purdell‐Lewis DJ (1982) The pattern of mineralization of traumatically‐induced developmental defects of sheep enamel assessed by microhardness and microradiography. J Dent Res 61, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Suckling GW, Nelson DGA, Patel MJ (1989) Macroscopic and scanning electron microscopic appearance and hardness values of developmental defects in human permanent tooth enamel. Adv Dent Res 3, 219–233. [DOI] [PubMed] [Google Scholar]
- Suga S (1989) Enamel hypomineralization viewed from the pattern of progressive mineralization of human and monkey developing enamel. Adv Dent Res 3, 188–198. [DOI] [PubMed] [Google Scholar]
- Sweeney EA, Saffir AJ, De Leon R (1971) Linear hypoplasia of deciduous incisor teeth in malnourished children. Am J Clin Nutr 24, 29–31. [DOI] [PubMed] [Google Scholar]
- Witzel C, Kierdorf U, Dobney K, et al. (2006) Reconstructing impairment of secretory ameloblast function in porcine teeth by analysis of morphological alterations in dental enamel. J Anat 209, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel C, Kierdorf U, Schultz M, et al. (2008) Insights from the inside: histological analysis of abnormal enamel microstructure associated with hypoplastic enamel defects in human teeth. Am J Phys Anthropol 136, 400–414. [DOI] [PubMed] [Google Scholar]
- Zanolli C, Grine FE, Kullmer O, et al. (2015) The early Pleistocene deciduous hominid molar FS‐72 from the Sangiran Dome of Java, Indonesia: a taxonomic reappraisal based on its comparative endostructural characterization. Am J Phys Anthropol 157, 666–674. [DOI] [PubMed] [Google Scholar]
- Zheng L, Seon YJ, Mourão MA, et al. (2013) Circadian rhythms regulate amelogenesis. Bone 55, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsigmondy O (1893) On congenital defects of the enamel. Dental Cosmos 35, 709–717. [PMC free article] [PubMed] [Google Scholar]


