Abstract
Background
Multiple factors determine children’s nutritional status, including energy and nutrient intake, recurrent infectious diseases, access (or lack thereof) to clean water and improved sanitation, and hygiene practices, among others. The “Vegetables go to School: improving nutrition through agricultural diversification” (VgtS) project implements an integrated school garden programme in five countries, including Burkina Faso. The aim of this study was to determine the prevalence of undernutrition and its risk factors among schoolchildren in Burkina Faso before the start of the project.
Methods
In February 2015, a cross-sectional survey was carried out among 455 randomly selected children, aged 8–14 years, in eight schools in the Plateau Central and Centre-Ouest regions of Burkina Faso. Nutritional status was determined by anthropometric assessment. Helminth and intestinal protozoa infections were assessed using the Kato-Katz and a formalin-ether concentration method. A urine filtration technique was used to identify Schistosoma haematobium eggs. Prevalence of anaemia was determined by measuring haemoglobin levels in finger-prick blood samples. Questionnaires were administered to children to determine their knowledge of nutrition and health and their related attitudes and practices (KAP). Questionnaires were also administered to the children’s caregivers to identify basic household socio-demographic and economic characteristics, and water, sanitation and hygiene (WASH) conditions. To determine the factors associated with schoolchildren’s nutritional status, mixed logistic regression models were used. Differences and associations were considered statistically significant if P-values were below 0.05.
Results
Complete datasets were available for 385 children. The prevalence of undernutrition, stunting and thinness were 35.1%, 29.4% and 11.2%, respectively. The multivariable analysis revealed that undernutrition was associated with older age (i.e. 12–14 years compared to <12 years; adjusted odds ratio (aOR) = 3.45, 95% confidence interval (CI) 2.12–5.62, P < 0.001), multiple pathogenic parasitic infections (aOR = 1.87, 95% CI 1.02–3.43, P = 0.044) and with moderate and severe anaemia in children (aOR = 2.52, 95% CI 1.25–5.08, P = 0.010).
Conclusions
We found high prevalence of undernutrition among the children surveyed in the two study regions of Burkina Faso. We further observed that undernutrition, anaemia and parasitic infections were strongly associated. In view of these findings, concerted efforts are needed to address undernutrition and associated risk factors among school-aged children. As part of the VgtS project, WASH, health education and nutritional interventions will be implemented with the goal to improve children’s health.
Trial registration
ISRCTN17968589 (date assigned: 17 July 2015).
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-016-0230-x) contains supplementary material, which is available to authorized users.
Keywords: Anaemia, Burkina Faso, Intestinal parasitic infections, School garden, Undernutrition, Water, sanitation, and hygiene (WASH)
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
In Burkina Faso, undernutrition, anaemia and diarrhoeal diseases are the leading causes of morbidity in children under the age of five. The most recent Demographic and Health Survey (DHS) of 2010 showed that 88% of children under five were anaemic, 35% were undernourished and 15% suffered from diarrhoea in the two weeks preceding the DHS [1]. While DHS and national nutrition surveillance systems in Burkina Faso have routinely measured the height and weight of children under the age of five since the early 1990s, there is a lack of national anthropometric data for school-aged children (5–14 years) [2–4].
The determinants of children’s nutritional status are multifactorial [5–7]. The direct causes of undernutrition in children are insufficient energy and nutrient intake, and recurrent infectious diseases (e.g. intestinal parasitic infection, malaria and diarrhoea) [7]. Factors that affect children’s nutritional status indirectly include a lack of access to clean water and improved sanitation, inadequate hygiene, a paucity of health education and, importantly, inappropriate agricultural practices and insufficiently healthy and diverse diets [5–9]. Low socio-economic and sanitary conditions prevail in Burkina Faso and together contribute to the burden of infectious diseases in children [1, 10, 11], further compromising their nutritional status [5–9, 12].
To address these challenges, research institutions and international development organisations are paying increased attention to enhancing synergies among agriculture, nutrition and health. The Sustainable Development Goals (SDGs) have recognised agriculture as a source of nutrition and well-being, as addressed in SDG 2: “End hunger, achieve food security and improved nutrition and promote sustainable agriculture” [13]. Yet, there is a dearth of evidence to support the effect of agricultural and health interventions on improving children’s nutritional status [14, 15]. To fill this research gap, a multi-country and multi-stakeholder project entitled “Vegetables go to School: improving nutrition through agricultural diversification” (VgtS), was developed to address schoolchildren’s nutrition in an interdisciplinary way, through introducing school vegetable gardens and other school-based health, nutritional and environmental interventions. The VgtS project is active in five countries in Africa and Asia (Bhutan, Burkina Faso, Indonesia, Nepal and the Philippines), with the overall goal of improving schoolchildren’s nutritional status [16]. Under the VgtS project, two intervention studies were implemented in Burkina Faso and Nepal. These studies assessed schoolchildren’s nutritional and health status at baseline and at 12 months follow-up, using a set of selected qualitative and quantitative indicators. The findings from these studies guided the development of complementary nutrition and water, sanitation and hygiene (WASH) interventions to operate alongside the school garden programme. Details of the study design and procedures have been described elsewhere [16].
The Burkina Faso setting provided an opportunity to understand the complex interactions among agriculture, undernutrition, intestinal parasitic infections and WASH conditions. Agriculture is a major source of livelihoods in the country and inadequate WASH conditions are well known risk factors for both undernutrition and intestinal parasitic infections [11, 17–20]. In this article, we report findings from a cross-sectional baseline survey carried out in Burkina Faso as part of the intervention component of the VgtS project.
Methods
Study area
We conducted a cross-sectional baseline study in February 2015. The schools participating in the VgtS project in Burkina Faso are located in the Plateau Central and the Centre-Ouest regions. The Plateau Central region is situated in the north-east, approximately 30–120 km from the capital, Ouagadougou. The Centre-Ouest region is located in the south-west, some 40–180 km from Ouagadougou (Fig. 1). The two regions are located in the semi-arid North-Sudanian zone, characterised by fields, bushes and scattered trees and a Sudano-Sahelien climate (a short wet and a long dry season, with annual precipitation of 600–1 000 mm).
Fig. 1.
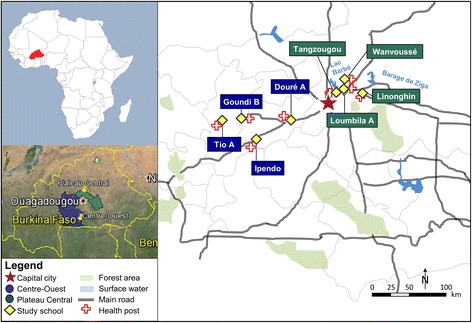
Study sites of the cross-sectional survey in Burkina Faso, February 2015
Sample size and sampling method
Our sample size calculation targeted the association between the prevalence of intestinal parasitic infection and the degree of risk among children, aged 8–14 years. We assumed a minimum prevalence of intestinal parasitic infections of 40%, with a coefficient of variation of 10% across schools and a proportion of high - risk children of 25%. We aimed for a power of 85% to detect a difference in infection rates (with P < 0.05) between high- and low-risk children at eight schools, for a true odds ratio (OR) of at least 2. A Monte Carlo simulation (5 000 iterations) provided a minimal sample size of 400 children (i.e. 50 children per school). Eight of the 30 VgtS project schools in Burkina Faso were randomly selected to participate in the study [16]. In each of the sampled schools, 55–60 children (boys and girls in ratio 1:1) were randomly selected; we assumed that the final sample size would be reduced by 15% due to non-response and missing data [16]. The inclusion criteria for this study were: (i) schoolchildren between the ages of 8 and 14 years; (ii) parents/guardians of the children providing written informed consent; and (iii) children additionally providing oral assent.
Anthropometric survey
Trained field staff collected anthropometric measurements from the children, using a height measuring board and a digital scale (Seca 877; Seca, Germany) with a precision of 0.1 cm and 0.1 kg, respectively and adhering to standard procedures [21]. Anthropometric indices were calculated in accordance with the World Health Organization (WHO) reference, using AnthroPlus (WHO; Geneva, Switzerland) [22, 23]. For children without an exact date of birth or whose age was unknown, school registration lists were consulted. If the exact month or date of birth was unavailable, anthropometric indices were calculated assuming 30 June (mid-year) as the child’s date of birth. Three anthropometric indices — height-for-age (HAZ, stunting), body mass index-for-age (BMIZ, thinness) and weight-for-age (WAZ, underweight) — were expressed as differences from the median in z-scores. Children were classified as stunted, thin, or underweight if z-scores of HAZ, BMIZ and WAZ were less than - 2 standard deviations (SD) below the WHO reference median of the standard population. WAZ was only used for children aged 8–10 years, as reference data were not available for children over 10 years [22, 23]. Children were classified as overweight if BMIZ was above 1 SD. We considered children to be malnourished when classified as stunted, thin, underweight or overweight; undernourished children were those classified as stunted, thin or underweight. The categories of stunting, thinness and underweight are not mutually exclusive, as these conditions often overlap; an undernourished child can, for example, be classified as stunted and thin, concurrently.
Haemoglobin survey
Haemoglobin (Hb) concentration was determined in finger-prick capillary blood samples, using a HemoCue portable device (HemoCue Hb 201 System; Ängelholm, Sweden) [24]. Children were classified as mildly anaemic if Hb concentration was less than 11.5 g/dl for children aged 8–11 years and less than 12 g/dl for children aged 12–14 years. Children were classified as moderately and severely anaemic if Hb concentration was less than 11 g/dl and 8 g/dl, respectively [25].
Parasitological survey
Children were asked to provide a fresh morning stool and a mid-morning post-exercise urine sample, collected on two consecutive days. Stool and urine samples were processed the same day by experienced laboratory technicians. From each stool, a single Kato-Katz thick smear was prepared for diagnosis of soil-transmitted helminths (Ascaris lumbricoides, hookworm and Trichuris trichiura), Schistosoma mansoni and other helminths. A formalin-ether concentration (FEC) technique was also performed on each sample to diagnose helminths and intestinal protozoa (Blastocystis hominis, Chilomastix mesnili, Endolimax nana, Entamoeba coli, Entamoeba histolytica/E. dispar, Entamoeba hartmanni, Giardia intestinalis, and Iodamoeba bütschlii) [26, 27]. Urine samples were examined for microhaematuria using reagent strips (Hemastix, Siemens Healthcare Diagnostics GmbH; Eschborn, Germany). A urine filtration technique was applied to detect the presence and number of S. haematobium eggs [28]. Helminth infection intensity was calculated based on criteria established by the WHO [29].
Questionnaire survey
Questionnaires were administered to children to determine their knowledge of nutrition and health and associated attitudes and practices (KAP) and to the caregivers to identify basic household socio-demographic and economic characteristics and WASH conditions. The KAP and household questionnaires were established according to international guidelines, using standardised questions amended by our research team [1, 30, 31]. Both questionnaires were pre-tested in the study area in November 2014, with children and caregivers who did not subsequently participate in the survey (as part of a pilot study carried out in different schools and villages, far away from those schools selected for the present study). Final local adaptations were made prior to the start of the survey in February 2015.
Data entry and storage
Data were double-entered in Excel 2010 (Microsoft; Redmond, USA). After removing inconsistencies, the datasets were combined and the accuracy of the merged database was verified against the original data through random cross-checking. Data were transferred to and stored electronically on a secure and password-protected server at the Swiss Tropical and Public Health Institute (Swiss TPH; Basel, Switzerland).
Statistical analysis
Categorical variables were described by absolute and relative frequencies. Numerical variables were described by their mean and SD if they were normally distributed, and by their median and interquartile range, otherwise. To characterise household socioeconomic status, we conducted a factor analysis. A list of recorded household assets were included, which took into account the construction materials of the house wall, roof and floor [32]. Four factors reflecting four different socioeconomic domains were retained, including; (i) housing wall materials; (ii) roof materials; (iii) floor materials; and (iv) main energy sources used.
To test for associations between undernutrition (including stunting, thinness and underweight) in children as an outcome variable and associated risk factors, we first conducted a univariable mixed logistic regression analysis with random intercepts at the level of the schools. We included random effects for schools in our logistic regression models, as outcomes might vary between schools due to local factors not accounted for in our models. Non-pathogenic, intestinal protozoa infections (Trichomonas intestinalis and E. coli) were excluded as potential risk factors for undernutrition in univariable and multivariable analysis. A new variable for hygiene behaviour was created using factor analysis with two conceptually similar categorical variables of: (i) mode of handwashing (e.g. handwashing with soap and water, with water only, with ash, and no handwashing); and (ii) handwashing frequency (before eating, after eating, after playing, and after defecation). Children were classified into one of three categories, reflecting poor, moderate or better hygiene behaviours.
Second, we used a multivariable mixed logistic regression model with random school intercepts and including the categorical exposure variables sex, age, project region and household socioeconomic status as additional independent variables. All other variables were added to the core model one by one, and those with a P < 0.2 (using likelihood ratio test) were included in the final multivariable model. ORs were reported to compare relative odds, while differences and associations were considered as statistically significant if P-values were below 0.05, and indicating a trend if P-values were between 0.05 and 0.1.
Statistical analyses were performed with Stata version 13 (StataCorp; College Station, USA). Maps, including geographical coordinates of the schools, were established in ArcMap™ version 10 (Environmental System Research Institute; Redlands, USA) and with the Google Earth™ mapping software (https://www.google.com/earth).
Results
Study compliance and respondents’ characteristics
Overall, 455 schoolchildren from eight schools were enrolled in the study. Figure 2 summarises study participation and compliance, from enrolment to the final sample included for statistical analysis.
Fig. 2.
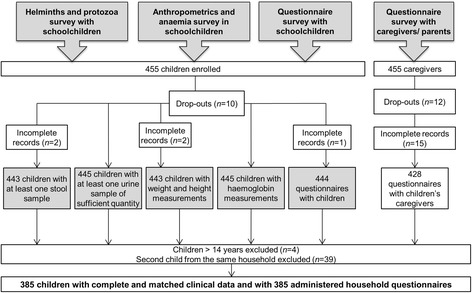
Participation in the different study groups of the cross-sectional survey in Burkina Faso, February 2015
Parasitological, anthropometric, Hb and KAP questionnaire data were linked by means of a unique identification code (ID). Erroneous ID codes or incomplete datasets with at least one of the parameters missing (e.g. anthropometrics, anaemia, urine and stool analyses, and child and household questionnaires) reduced the number of complete datasets from 455 to 424 children’s records and 385 corresponding household records for subsequent analyses. For households with more than one participating child, one child was selected at random for analysis; hence, another 39 children were excluded and our final dataset comprised 385 children from 385 unique households.
The mean age of children interviewed was 11 years (SD 0.7 years, range: 8–14 years). The mean age of the children’s caregivers interviewed was 45 years (SD 14.2 years, range: 20–95 years). Three-quarters of the children’s caregivers had not received any formal education, whereas 59 (15.3%) attended primary school and the remaining 38 (9.9%) received at least a secondary level of education. Almost 90% of children’s caregivers work in the agricultural sector. Respondents’ demographic and economic characteristics are summarised in Table 1.
Table 1.
Characteristics of the study population in the Plateau Central and Centre-Ouest regions, Burkina Faso, February 2015
| Children’s demographic characteristics | Number | Percent | |
|---|---|---|---|
| Age of childrena | |||
| Girls | 188 | 48.8 | |
| Boys | 197 | 51.2 | |
| Age group 1 (8–11 year) | 251 | 65.2 | |
| Age group 2 (12–14 years) | 134 | 34.8 | |
| Caregivers’ demographic and educational characteristics | |||
| Caregivers’ ageb | |||
| No formal schooling | 288 | 74.8 | |
| Primary education | 59 | 15.3 | |
| Secondary or higher education | 38 | 9.9 | |
| Main occupation of head of household | |||
| Agriculture | 344 | 89.4 | |
| Merchant | 8 | 2.1 | |
| Civil service | 9 | 2.3 | |
| No employment | 2 | 0.5 | |
| Others (housework or retirement) | 22 | 5.7 | |
| Socioeconomic domains | |||
| Roof material | Simple (natural and baked clay) | 37 | 9.6 |
| Metal cover | 348 | 90.4 | |
| Wall material | Simple (natural clay) | 359 | 93.3 |
| Baked or cemented clay | 26 | 6.7 | |
| Floor material | Simple (clay, sand, mud, straw) | 255 | 66.2 |
| Baked or cemented clay | 130 | 33.8 | |
| Energy used | Simple (charcoal, firewood) | 376 | 97.7 |
| Electricity and gas | 9 | 2.3 | |
a = mean age of 11.0 (±0.7) years
b = mean age of 45.0 (±14.2) years
Prevalence of malnutrition
Table 2 shows the extent of malnutrition, stratified by anthropometric indicators, including age, sex and region. The prevalence of malnutrition and undernutrition in this study were high, at 37.1% and 35.1%, respectively. The prevalence of stunting was 29.4%, while 11.2% of the children were classified as thin. Three out of the 55 children under the age of 10 years were underweight, while eight children were classified as overweight.
Table 2.
Prevalence of total and specific malnutrition indicators in schoolchildren, Burkina Faso, February 2015
| Variable | Malnutrition [n (%)] | Undernutrition [n (%)] | Stuntinga
[n (%)] |
Thinnessa
[n (%)] |
Underweighta [n (%)] | Overweightb [n (%)] | Anaemiac [n (%)] |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female (188) | 61 (32.5) | 57 (30.3) | 47 (25.0) | 24 (12.8) | 2 (1.1) | 4 (2.1) | 53 (28.2) |
| Male (197) | 82 (41.6) | 78 (39.6) | 66 (33.5) | 19 (9.6) | 1 (0.5) | 4 (2.0) | 57 (28.9) |
| Age group | |||||||
| 8–11 year (251) | 69 (27.5) | 61 (24.3) | 47 (18.7) | 16 (6.4) | 3 (1.2) | 8 (3.2) | 55 (21.9) |
| 12–14 years (134) | 74 (55.2) | 74 (55.2) | 66 (49.3) | 27 (20.2) | NA d | 0 (0) | 55 (41.0) |
| Region | |||||||
| Plateau Central (198) | 69 (34.9) | 64 (32.3) | 50 (25.3) | 19 (9.6) | 2 (1.0) | 5 (2.5) | 53 (26.8) |
| Centre-Ouest (187) | 74 (39.6) | 71 (38.0) | 63 (33.7) | 24 (12.8) | 1 (0.5) | 3 (1.6) | 57 (30.5) |
| Total | 143 (37.1) | 135 (35.1) | 113 (29.4) | 43 (11.2) | 3 (0.8) | 8 (2.1) | 110 (28.6) |
a z-score < − 2
b z-score > 1
c The category of anaemia includes all children classified as anaemic (mild, moderate and severe) based on the concentrations of haemoglobin (Hb) determined in a finger prick blood sample. The cut-offs for anaemia are age-specific: Hb <11.5 g/dl for children aged 8–11 years, and Hb <12 g/dl for children aged 12–14 years
d NA not available
Intestinal parasitic and Schistosoma infections
Table 3 shows differences in the prevalence of intestinal protozoa, faecal-oral transmitted helminths and Schistosoma infections in children, stratified by sex, age and region. We found that 86.2% of the children were infected with at least one intestinal parasite. Intestinal protozoa infections were highly prevalent (84.7%). Entamoeba histolytica/E. dispar was the predominant intestinal protozoon species (66.5%), followed by E. coli (37.4%), G. intestinalis (28.1%) and T. intestinalis (23.4%).
Table 3.
Prevalence of helminths and intestinal protozoa infections in schoolchildren, Burkina Faso, February 2015
| Variable | Trematodes | Total schistosomiasisa
[n (%)] |
Nematodes | Cestodes | Total faecal-oral transmitted helminthsc
[n (%)] |
Protozoa | Total protozoa [n (%)] |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. haematobium
a
[n (%)] |
S. mansoni
a
[n (%)] |
Hookworm [n (%)] |
H. nana
b
[n (%)] |
Entamoeba histolytica/E. dispar
[n (%)] |
Entamoeba coli
[n (%)] |
Giardia intestinalis
[n (%)] |
Trichomonas intestinalis
[n (%)] |
Balantidium coli
[n (%)] |
||||
| Sex | ||||||||||||
| Female (188) | 7 (3.7) | 0 (0) | 7 (3.7) | 0 (0) | 11 (5.9) | 11 (5.9) | 131 (69.7) | 67 (35.6) | 44 (23.4) | 39 (20.7) | 1 (0.5) | 161 (85.6) |
| Male (197) | 8 (4.1) | 1 (0.5) | 9 (4.6) | 3 (1.5) | 14 (7.1) | 16 (8.1)c | 125 (63.5) | 77 (39.1) | 64 (32.5) | 51 (25.9) | 0 (0) | 165 (83.8) |
| Age group | ||||||||||||
| 8–11 year (251) | 8 (3.2) | 0 (0) | 8 (3.2) | 2 (0.8) | 13 (5.2) | 15 (6.0) | 163 (64.9) | 93 (37.1) | 69 (27.5) | 51 (20.3) | 0 (0) | 209 (83.3) |
| 12–14 years (134) | 7 (5.2) | 1 (0.8) | 8 (6.0) | 1 (0.8) | 12 (9.0) | 12 (9.0)c | 93 (69.4) | 51 (38.1) | 39 (29.1) | 39 (29.1) | 1 (0.8) | 117 (87.3) |
| Region | ||||||||||||
| Plateau Central (198) | 8 (4.0) | 0 (0) | 8 (4.0) | 1 (0.5) | 5 (2.5) | 6 (3.0) | 110 (55.6) | 65 (32.8) | 49 (24.8) | 55 (27.8) | 0 (0) | 157 (79.3) |
| Centre-Ouest (187) | 7 (3.7) | 1 (0.5) | 8 (4.3) | 2 (1.1) | 20 (10.7) | 21 (11.2)c | 146 (78.1) | 79 (42.3) | 59 (31.6) | 35 (18.7) | 1 (0.5) | 169 (90.4) |
| Total (385) | 15 (3.9) | 1 (0.3) | 16 (4.2) | 3 (0.8) | 25 (6.5) | 27 (7.0) | 256 (66.5) | 144 (37.4) | 108 (28.1) | 90 (23.4) | 1 (0.3) | 326 (84.7) |
a Schistosoma haematobium, Schistosoma mansoni
b Hymenolepis nana
c The category of total faecal-oral transmitted helminths includes children infected with hookworm and Hymenolepis nana. There is one child co-infected with hookworm and Hymenolepis nana.
Faecal-oral transmitted helminth infections were found in 7.0% of the children. Hymenolepis nana was the most frequently occurring species (6.5%). Only three children were infected with hookworm (0.8%). One child had a dual-species infection with hookworm and H. nana. Fifteen children were infected with S. haematobium (3.9%), while one child was infected with S. mansoni (0.3%).
Co-infections were common, affecting 32.5% of the children, whilst 15.6% and 4.7% suffered from triple and quadruplicate infections, respectively. Infections with H. nana, S. haematobium, hookworm and S. mansoni were of light intensity. The prevalence of intestinal protozoa and faecal-oral transmitted helminth infections differed significantly between schoolchildren in the Plateau Central region and those in Centre-Ouest (P < 0.05).
Prevalence of anaemia
The mean Hb concentration was 12.3 g/dl (SD 0.7 g/dl). The prevalence of anaemia in our study sample was 28.6% (Table 2). Few children were found to be severely anaemic (0.8%), while 11.2% were found to be moderately anaemic and 16.6% mildly anaemic.
Results from the questionnaire surveys
Key results from children’s nutrition and health KAP survey and from the household questionnaire are summarised in Table 4. While 79.7% of the children reported using latrines at school for defecation, 22.1% reported washing their hands after defecation. Most children (87.8%) reported washing their hands before eating and 7.3% after playing. Four out of five (79.5%) children reported using soap and water to wash their hands. Combining the mode and frequency of handwashing, children were divided into one of three hygiene categories: 14.6% in the lower, 59.0% in the middle and 26.4% in the better hygiene category. Among the households participating in our survey, 55.3% did not own a latrine, while 23.1% had access to an improved latrine. The majority of children (82.1%) and 22.1% of their caregivers stated that they had never heard of malnutrition. Of the interviewed caregivers, 96.9% indicated that their participating child was breastfed.
Table 4.
Key findings from children’s nutrition and health KAP survey and household questionnaire in Burkina Faso, February 2015
| Children (n = 385) | Number | Percent |
| Selected KAPa indicators: | ||
| Handwashingb | ||
| Water only | 344 | 89.4 |
| Water and soap | 306 | 79.5 |
| With ash | 12 | 3.1 |
| With mud | 1 | 0.3 |
| Before eating | 338 | 87.8 |
| After eating | 55 | 14.3 |
| After playing | 28 | 7.3 |
| After defecation | 85 | 22.1 |
| Do not wash hands | 16 | 4.2 |
| Hygiene behaviourc | ||
| Lower category (1) | 56 | 14.6 |
| Middle score (2) | 227 | 59.0 |
| Best category (3) | 102 | 26.4 |
| Sanitary behaviour at school | ||
| Using latrines at school | 307 | 79.7 |
| Open defecation (fields, bush) | 71 | 18.5 |
| Others (at home, teachers home) | 7 | 1.8 |
| Meals (day prior to the survey) | ||
| Breakfast | 330 | 85.7 |
| Lunch | 351 | 91.2 |
| Dinner | 358 | 93.0 |
| Nutritional knowledge | ||
| Heard about malnutrition | 69 | 17.9 |
| Households (n = 385) | Number | Percent |
| Household WASHd characteristics | ||
| Availability of soap (observational) | 118 | 30.7 |
| Type of latrines used | ||
| Flush toilet (i) | 0 | 0 |
| VIP latrinee (ii) | 14 | 3.6 |
| Traditional pit latrine (iii) | 83 | 21.6 |
| EcoSanf (iv) | 60 | 15.6 |
| Samplat latrine (v) | 15 | 3.9 |
| No facilities/open defecation (vi) | 213 | 55.3 |
| Total improvedg (i, ii, iv, v) | 89 | 23.1 |
| Total unimprovedh (iii, vi) | 296 | 76.9 |
| Nutritional knowledge and practices | ||
| Heard about malnutrition | 300 | 77.9 |
| Participating child was breastfed | 373 | 96.9 |
a Knowledge, attitudes and practices
b Multiple responses occurred for the variables characterising the mode (how) and frequency (when) of handwashing.
c A new variable for hygiene behaviour was created using factor analysis with two conceptually similar categorical variables of: (i) mode of handwashing (handwashing with water and soap, with water only, with ash, no handwashing); and (ii) its frequency (before eating, after eating, after playing, and after defecation). Children were classified into three categories with lower, middle and better hygiene behaviours.
d Water, sanitation and hygiene
e Ventilated improved pit (VIP) latrine is an improved type of pit latrine, which helps remove odours and prevent flies from breeding and escaping. Excreta are collected in a dry pit which has a vent pipe covered with a fly-proof screen at the top
f Ecological sanitation (EcoSan) toilets are linked to a closed system that does not need water. The toilet is based on the principle of safely recycling excreta resources to create a valuable resource for agriculture
g The total improved sanitation category includes sanitation facilities that hygienically separate human excreta from human contact. In our study, these were: (i) flush toilet, (ii) VIP latrine, (iv) EcoSan toilets, and (v) latrine with slab
h The total unimproved sanitation category in our study included: (iii) traditional pit latrines, and (vi) no facilities/open defecation)
Results from the logistic regression analysis
Table 5 provides an overview of the associations between undernutrition and all measured helminth and pathogenic intestinal protozoa infections, nutrition and health KAP, caregivers’ socioeconomic characteristics and WASH conditions observed in univariable and multivariable regression analyses. The prevalence of undernutrition significantly differed between age groups, with the older age group (12–14 years) showing significantly higher odds of undernutrition (aOR = 3.45, 95% CI 2.12–5.62, P < 0.001). Girls showed lower odds of being undernourished, but this association lacked statistical significance in the multivariable analysis. No significant association was observed between undernutrition and study region (P > 0.05).
Table 5.
Results from univariable and multivariable logistic regression analysis with undernutrition as outcome
| Undernutrition N = 385 / N(cases) = 135 |
Univariable logistic regressiona | Multivariable logistic regressionb | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | aOR | 95% CI | P | |||
| Sex | Male | 1.00 | ||||||
| Female | 0.70 | 0.45–1.09 | 0.112 | 0.72 | 0.46–1.14 | 0.163 | ||
| Age group | 8–11 year | 1.00 | ||||||
| 12–14 years | 3.57 | 2.20–5.78 | <0.001 | 3.45 | 2.12–5.62 | <0.001 | ||
| Region | Centre-Ouest | 1.00 | ||||||
| Plateau Central | 0.89 | 0.35–2.27 | 0.804 | |||||
| Multiple pathogenic parasites | ”yes” vs. “no” | 1.94 | 1.09–3.47 | 0.025 | 1.87 | 1.02–3.43 | 0.044 | |
| Intestinal pathogenic protozoa | “yes” vs. “no” | 1.78 | 1.03–3.06 | 0.039 | 1.71 | 0.97–3.03 | 0.064 | |
| Hymenolepis nana | “yes” vs. “no” | 1.42 | 0.60–3.36 | 0.425 | ||||
| Schisotosoma haematobium | “yes” vs. “no” | 0.76 | 0.22–2.56 | 0.659 | ||||
| Giardia intestinalis | “yes” vs. “no” | 1.44 | 0.90–2.32 | 0.131 | 1.46 | 0.89–2.40 | 0.133 | |
| Entamoeba histolytica/E. dispar | “yes” vs. “no” | 1.39 | 0.85–2.25 | 0.187 | 1.41 | 0.85–2.34 | 0.184 | |
| Anaemia | No | 1.00 | ||||||
| Mild | 1.59 | 0.89–2.85 | 0.121 | 1.24 | 0.67–2.31 | 0.486 | ||
| Moderatec | 2.89 | 1.48–5.64 | 0.002 | 2.52 | 1.25–5.08 | 0.010 | ||
| Middle score (2) | 1.00 | |||||||
| Hygiened | Lower category (1) | 1.15 | 0.59–2.25 | 0.676 | ||||
| Best category (3) | 1.36 | 0.82–2.25 | 0.233 | |||||
| Sanitary behaviour at school | Open defecatione | 1.00 | ||||||
| Using latrines at school | 0.97 | 0.48–1.95 | 0.922 | |||||
| Others (at teachers’) | Na | |||||||
| Household sanitary conditions | Improved latrines | 1.00 | ||||||
| No latrines/open defecation | 0.96 | 0.54–0.54 | 0.886 | |||||
| Traditional latrine | 1.18 | 0.60–2.29 | 0.634 | |||||
| Availability of soap | “yes” vs. “no” | 1.14 | 0.70–1.84 | 0.599 | ||||
| Child’s eating habits (day prior to the survey) | Breakfast | “no vs. yes”f | 0.72 | 0.38–1.38 | 0.326 | |||
| Lunch | “no vs. yes”f | 1.88 | 0.89–4.00 | 0.100 | 1.52 | 0.69–3.32 | 0.298 | |
| Dinner | “no vs. yes”f | 1.30 | 0.57–2.99 | 0.534 | ||||
| Child “heard about malnutrition” | “no vs. yes”f | 1.11 | 0.64–1.95 | 0.709 | ||||
| Caregiver “heard about malnutrition” | “no vs. yes”f | 1.14 | 0.67–1.94 | 0.618 | ||||
| “Breastfed child” | “no vs. yes”f | 2.20 | 0.41–11.71 | 0.354 | ||||
| Caregiver’s education | Never went to school | 1.00 | ||||||
| Primary education | 1.30 | 0.71–2.37 | 0.390 | |||||
| Secondary education | 0.87 | 0.40–1.89 | 0.716 | |||||
| Caregiver’s occupation | Agriculture | 1.00 | ||||||
| Civil service | 0.35 | 0.04–3.01 | 0.341 | |||||
| Merchant | 0.35 | 0.33–5.23 | 0.702 | |||||
| Othersg | 0.71 | 0.28–1.85 | 0.487 | |||||
a P-value and odds ratio (OR) based on likelihood ratio test. In univariable logistic regression, the overall P-value of the models is indicated in bold letters
b P-value and adjusted (a) OR based on likelihood ratio test of the multivariable regression model. The mixed multivariable logistic regression model with random school intercepts included the categorical exposure variables sex, age group, socioeconomic domains and project region. All risk factors that had a P-value lower than 0.2 in the univariable analyses were included into the multivariable regression analysis (as indicated in the table)
c The category of moderate anaemia includes the severely anaemic children (n = 3)
d This variable was created with two conceptually similar categorical variables of: (i) mode of handwashing (handwashing with soap and water, with water only, with ash, no handwashing); and (ii) handwashing frequency (before eating, after eating, after playing, and after defecation) where multiple responses were possible. Children were classified into one of three categories, with lower, middle and better hygiene behaviours
e Open defecation includes the category of defaecating in the bush and behind the latrines
f The reference category for the OR is “yes” as compared to “no”
g ‘Others’ includes homemakers, retirees and unemployed people
Children infected with multiple pathogenic parasites and those with moderate - to - severe anaemia, were at significantly higher odds of being undernourished (aOR = 1.87, 95% CI 1.02–3.43, P = 0.044; and aOR = 2.52, 95% CI 1.25–5.08, P = 0.010, respectively).
Overall, children with better hygiene behaviours (third category) did not show lower odds for undernutrition than those in the middle or lower hygiene categories (P > 0.5). Relying on traditional pit latrines or having no toilet facility at home was not associated with increased odds for undernutrition in children. Moreover, children who reported not having eaten lunch the day prior to the survey and children who were not breastfed showed higher odds of undernutrition, but these associations were not statistically significant (P > 0.05). Neither the level of education of the children’s caregivers nor their occupation showed any statistically significant association with undernutrition.
Discussion
This paper presents findings from a cross-sectional survey on the prevalence of undernutrition and associated risk factors among schoolchildren, aged 8–14 years, from eight schools in the Plateau Central and Centre-Ouest regions of Burkina Faso. We found that undernutrition was highly prevalent among the surveyed children. Approximately a third of the children were undernourished (35.1%).
According to a study conducted in Ouagadougou in 2008/09 for the WHO’s “Nutrition Friendly School Initiative” (NFSI), the prevalence of stunting in schoolchildren (mean age of 11.5 years) was 8.8%, which is considerably lower than the prevalence of stunting among schoolchildren found in this study (29.4%) [33]. The proportion of thinness in children in our study was 11.2%, which is, however, comparable with the 13.7% found in the NFSI study [33]. Overweight children accounted for 2.1% of all children, with a higher incidence among children aged 8–11 years than among the older age group (3.2% vs. 0%), which is similar to the 2.3% reported in the NFSI study [33].
While few children were classified as thin, a considerably higher proportion of children in our study were stunted. Thinness is often associated with short-term risk factors, like seasonal climatic variations (which cause food scarcity/shortages) and increased occurrence of illnesses [34]. Our study was conducted in the post-harvest (mid-dry) season (February), before the commencement of the dry season (March-June) [35], suggesting that the cause of undernutrition was mainly of a chronic nature, associated with long-term risk factors.
The findings from multivariable mixed logistic regression analyses demonstrated a considerably higher risk of undernutrition among children older than 12 years of age. These results are in accordance with other studies, showing a higher prevalence of stunting in older children in low-income countries in Asia and Africa [36–38]. Moreover, children with moderate and severe anaemia (combined category) and with multiple helminths and intestinal pathogenic protozoa infections (“multiple pathogenic parasites”) showed significantly higher odds for undernutrition. Undernutrition and intestinal parasitic infections are intrinsically linked. While undernutrition and inadequate dietary intake lead to weight loss and weakened immunity and render a child more susceptible to infections, parasitic infections contribute to growth stunting by causing a vicious cycle of reduced food intake (loss of appetite), diarrhoea, malabsorption and/or increased nutrient wastage [39–41]. The observed association was statistically significant in our study, reinforcing evidence of the frequent coexistence of these conditions among children [40]. Moreover, while anaemia contributed to higher odds of undernutrition among children in our study, the aetiology of anaemia is multifactorial and can result from nutritional deficiencies and parasitic infections, among other things, which have been closely connected to the nutritional status of African schoolchildren [42–45].
Our questionnaire survey revealed important inadequacies in nutrition- and health-related knowledge and practices, but no clear association between undernutrition and WASH conditions or nutritional and health KAPs.
Our study has three main limitations. First, the findings presented here cannot be generalised for all of Burkina Faso. Despite the random selection of schools with a sample size large enough for children in this age range, the results are only representative of two regions. Second, the anthropometric survey has certain limitations with respect to the inaccuracy of children’s dates of birth. Indeed, we noted that a considerable number of children had their birthdays either on 31 December or on 1 January, according to the existing school records. Upon further probing in the interview, the children often did not know their exact date of birth. Hence, for these children, we took a mid-year point as the date of birth [46]. Third, only one single Kato-Katz thick smear and FEC from two stool samples from two consecutive days were examined for each participant. Our results may therefore underestimate the true prevalence of parasitic infections, due to the low sensitivity of the Kato-Katz technique and urine concentration method [47, 48].
Despite these limitations, our findings highlight a number of important issues. First, undernutrition in schoolchildren in this part of Burkina Faso is highly prevalent. We therefore suggest giving greater attention to the overall nutritional status of school-aged children. So far, comprehensive population-based data, such as the DHS, focus on adolescents over the age of 15 years for sexual and reproductive health issues or on children under 5 years of age, as they are more vulnerable and prone to disease, illness and death [1, 49–51]. Children under five are often the primary focus of strategies and actions to address malnutrition [7, 52, 53]. Despite the increased odds of survival for children after the age of five (they generally have a lower prevalence of infections when compared to children under the age of five), school-aged children have increased nutritional needs to support the adolescent growth spurt, requiring diets rich in energy and micronutrients and sufficient in both quantity and quality [54]. It is therefore crucial to address the nutritional needs of children in this age group to match their growth requirements [55].
Second, the results of our study highlight the need for a more profound understanding of how helminths and other intestinal parasites mediate pathways to undernutrition. In particular, it is important to investigate other primary factors related to the burden of undernutrition among school-aged children, such as malaria and other parasitic infections, and the bioavailability and absorption of micronutrients so as to prevent long-term effects of undernutrition [56–58].
To address the factors underlying and contributing to schoolchildren’s nutritional status, we support the growing recommendation from several agencies to enhance multidisciplinary strategies and programmes, including nutrition and WASH interventions for school-aged children, in order to ensure optimal health, growth and development continuing after the age of five [59–61]. Such measures should be reflected in the current development of targets and indicators for reaching SDG 2.
Conclusions
This study provides new insight into the burden of undernutrition and its risk factors among schoolchildren in Burkina Faso, a country that lacks data on the health of children, aged 8–14 years. Our study shows that undernutrition is highly prevalent in the eight schools of the Plateau Central and Centre-Ouest regions (32.3 and 38.0%, respectively) of Burkina Faso. We also observed that undernutrition, anaemia and parasitic infections were strongly associated. In view of these findings, concerted efforts are needed to address undernutrition and the associated risk factors among school-aged children. As part of the VgtS project, WASH, health education and nutritional interventions will be implemented with the goal of improving schoolchildren's health.
Acknowledgements
We would like to thank all of the study participants for their commitment, the national and district health authorities for their kind support and interest, and the team at the Institute for Health Sciences Research (IRSS) in Burkina Faso for their support and technical assistance during the field work and laboratory investigation. We also specifically thank our field team for their efforts in data collection and for their skilled stool and urine examination.
We appreciate the institutional involvement of the Ministry of Health in Burkina Faso. We are grateful to our project partners from the “Vegetables go to School” project; namely, the AVRDC-World Vegetable Centre (Shanua, Taiwan) and the University of Freiburg (Freiburg, Germany), for their valuable support. This study received financial support from the Swiss Agency for Development and Cooperation.
Funding
This work is part of the ‘Vegetables go to School’ research project (Collaborative Project); supported by the Swiss Agency for Development and Cooperation under grant agreement contract number 81024052 (project 7 F-08511.01). The funder had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The dataset supporting the conclusions of this article will not be shared. The paper is written as part of the academic degree of a PhD and therefore the data will be used exclusively by the author.
Authors’ contribution
All listed authors contributed to the study design. SE, AMK and SD coordinated the field and laboratory work. TG supervised the laboratory technicians and assisted in data collection with BS. SE and AMK supervised the research assistants. SE performed the statistical analysis under the supervision of CS and drafted the manuscript. AMK, SD, PO, JG, AS, CS, JU and GC contributed to the interpretation of the data, manuscript writing and revisions. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the “Ethikkommission Nordwest-und Zentralschweiz” in Switzerland (EKNZ, reference no. 2014–161) and by the “Comité d’Ethique pour la Recherche en Santé, Ministère de la Recherche Scientifique et de l’Innovation, et Ministère de la Santé” (reference no. 2015-02-026). The study is registered with the clinical trial registry ISRCTN (identifier: ISRCTN17968589). Community and school awareness-raising activities entailed holding two meetings in a classroom at each school; one four weeks prior to the study and one on the day of the study. The purpose of these meetings was to discuss the objectives, procedures, potential benefits and risks of the study with district educational authorities, school directors, teachers, parents and community representatives. Informed consent (via signature) was obtained from the child’s parents or guardians. For illiterate parents/guardians, a fingerprint was obtained in the presence of a literate witness from the school (principal or teacher), whilst children assented orally. It was emphasised that participation was voluntary and that children could withdraw at any time without further obligation. All data records were anonymised, provided with a personal identifier and kept confidential.
Results were communicated to participants. Those found with mild or moderate anaemia (Hb <11.5 g/dl for children aged 8–11 years and Hb <12 g/dl for children aged 12–14 years) were referred to a local health centre and treated with iron supplements for 40 days, free of charge. Children found with severe anaemia (Hb < 8 g/dl) and severely malnourished children were referred to a local health centre for further investigation, following national guidelines [62, 63]. Children infected with any kind of intestinal protozoa or helminth were treated according to national guidelines (i.e., a 15–50 mg/kg single dose of metronidazole for five consecutive days against intestinal protozoa infection, a triple dose of 400 mg albendazole against soil-transmitted helminth infections, a 40 mg/kg single dose praziquantel against schistosomiasis and four tablets of niclosamide of 500 mg in two doses for six consecutive days to treat Hymenolepis nana). Trained teachers, in collaboration with our research team, and local health personnel, with close involvement of the parents/guardians of infected children, administered anti-parasitic medications and carefully observed children for proper medication intake and adverse events. All treatments were provided free of charge.
Abbreviations
- aOR
Adjusted odds ratio
- BMIZ
Body mass index-for-age
- CI
Confidence interval
- DHS
Demographic and Health Survey
- EKNZ
Ethikkommission Nordwest- und Zentralschweiz
- FEC
Formalin-ether concentration
- HAZ
Height-for-age
- Hb
Haemoglobin
- ID
Identification code
- IRSS
Institute for Health Sciences Research
- KAP
Knowledge, attitudes and practices
- NFSI
Nutrition Friendly School Initiative
- SD
Standard deviation
- SDGs
Sustainable Development Goals
- Swiss TPH
Swiss Tropical and Public Health Institute
- VgtS
Vegetables go to School: improving nutrition through agricultural diversification
- WASH
Water, sanitation and hygiene
- WAZ
Weight-for-age
- WHO
World Health Organization
Additional file
Multilingual abstracts in the five official working languages of the United Nations. (PDF 661 kb)
References
- 1.INSD and ICF International . Enquête Démographique et de Santé et à Indicateurs Multiples du Burkina Faso 2010. Calverton: Institut National de la Statistique et de la Démographie and ICF International; 2012. [Google Scholar]
- 2.WHO . World health statistics 2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 3.Friedman G. Review of national nutrition surveillance systems. Washington: Food and Nutrition Technical Assistance III Project (FANTA); 2014. [Google Scholar]
- 4.IFPRI . Global nutrition report 2015: actions and accountability to advance nutrition and sustainable development. Washington: International Food Policy Research Institute; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 6.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–54. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNICEF . Improving child nutrition: the achievable imperative for global progress. New York: United Nations Children's Fund; 2013. [Google Scholar]
- 8.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–5. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 9.World Bank . Improving nutrition through multisectoral approaches. Washington: World Bank; 2013. [Google Scholar]
- 10.UNICEF and WHO . Progress on drinking water and sanitation. Joint Monitoring Programme update 2015. New York; Geneva: United Nations Children's Fund; World Health Organization; 2015. [Google Scholar]
- 11.Institute for Health Metrics and Evaluation. Global Burden of Disease compare and visualisation. Seattle: University of Washington; 2013. http://vizhub.healthdata.org/gbd-compare/. Accessed 31 Aug 2016.
- 12.WHO . Guidelines for drinking-water quality. Geneva: World Health Organization; 2011. [Google Scholar]
- 13.United Nations . Sustainable Development Goals. 2015. [Google Scholar]
- 14.Thompson B, Amoroso L. FAO’s approach to nutrition-sensitive agricultural development. Rome; Geneva: Food and Agriculture Organization; World Health Organization; 2011. [Google Scholar]
- 15.Waage J, Hawkes C, Turner R, Ferguson E, Johnston D, Shankar B, et al. Current and planned research on agriculture for improved nutrition: a mapping and a gap analysis. Proc Nutr Soc. 2013;72:E316.
- 16.Erismann S, Shrestha A, Diagbouga S, Knoblauch A, Gerold J, Herz R, et al. Complementary school garden, nutrition, water, sanitation and hygiene interventions to improve children's nutrition and health status in Burkina Faso and Nepal: a study protocol. BMC Public Health. 2016;16:244. doi: 10.1186/s12889-016-2910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prüss-Üstün A, Bos R, Gore F, Bartram J. Safer water, better health: costs, benefits and sustainability of interventions to protect and promote health. Geneva: World Health Organization; 2008. [Google Scholar]
- 18.Speich B, Croll D, Fürst T, Utzinger J, Keiser J. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:87–99. doi: 10.1016/S1473-3099(15)00349-7. [DOI] [PubMed] [Google Scholar]
- 19.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FAO . Country fact sheet on food and agriculture policy trends. Rome: Food and Agriculture Organization; 2014. [Google Scholar]
- 21.WHO. Growth reference 5–19 years. Geneva: World Health Organization; 2016. http://www.who.int/growthref/en/. Accessed 12 Aug 2016.
- 22.De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . AnthroPlus for personal computers manual: Software for assessing growth of the world's children and adolescents. Geneva: World Health Organization; 2009. [Google Scholar]
- 24.WHO . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 25.WHO . Iron deficiency anaemia. Assessment, prevention and control. A guide for programme managers. Geneva: World Health Organization; 2001. [Google Scholar]
- 26.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 27.Utzinger J, Botero-Kleiven S, Castelli F, Chiodini PL, Edwards H, Kohler N, et al. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin Microbiol Infect. 2010;16:267–73. doi: 10.1111/j.1469-0691.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- 28.Utzinger J, N'Goran EK, Caffrey CR, Keiser J. From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011;120(Suppl 1):S121–37. doi: 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 29.WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committe. WHO Tech Rep Ser. 2002;912:1–57. [PubMed]
- 30.Marías Y, Glasauer P. Guidelines for assessing nutrition-related knowledge, attitudes and practices. Rome: Food and Agriculture Organization of the United Nations; 2014. [Google Scholar]
- 31.Helen Keller Interational. HKI Questionnaire de base. 2010. http://gaap.ifpri.info/files/2014/08/HKI_village_FINALE.pdf. Accessed 21 July 2016.
- 32.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 33.Dabone C, Delisle HF, Receveur O. Poor nutritional status of schoolchildren in urban and peri-urban areas of Ouagadougou (Burkina Faso). Nutr J. 2011;10:34. [DOI] [PMC free article] [PubMed]
- 34.Egata G, Berhane Y, Worku A. Seasonal variation in the prevalence of acute undernutrition among children under five years of age in east rural Ethiopia: a longitudinal study. BMC Public Health. 2013;13:864. doi: 10.1186/1471-2458-13-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra A, Hansen JW, Dingkuhn M, Baron C, Traoré SB, Ndiaye O, et al. Sorghum yield prediction from seasonal rainfall forecasts in Burkina Faso. Agr Forest Meteorol. 2008;148:1798–814. doi: 10.1016/j.agrformet.2008.06.007. [DOI] [Google Scholar]
- 36.Mushtaq MU, Gull S, Khurshid U, Shahid U, Shad MA, Siddiqui AM. Prevalence and socio-demographic correlates of stunting and thinness among Pakistani primary school children. BMC Public Health. 2011;11:790. doi: 10.1186/1471-2458-11-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lwanga F, Kirunda BE, Orach CG. Intestinal helminth infections and nutritional status of children attending primary schools in Wakiso district, Central Uganda. Int J Environ Res Public Health. 2012;9:2910–21. doi: 10.3390/ijerph9082910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degarege D, Degarege A, Animut A. Undernutrition and associated risk factors among school age children in Addis Ababa, Ethiopia. BMC Public Health. 2015;15:375. doi: 10.1186/s12889-015-1714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephenson LS, Holland C. Controlling intestinal helminths while eliminating lymphatic filariasis. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 40.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–8. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 41.Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites—should we use a holistic approach? Int J Infect Dis. 2010;14:e732–8. doi: 10.1016/j.ijid.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 42.Bhargava A, Jukes M, Lambo J, Kihamia CM, Lorri W, Nokes C, et al. Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food Nutr Bull. 2003;24:332–42. doi: 10.1177/156482650302400403. [DOI] [PubMed] [Google Scholar]
- 43.Brooker S, Clements ACA, Hotez PJ, Hay SI, Tatem AJ, Bundy DAP, et al. The co-distribution of Plasmodium falciparum and hookworm among African schoolchildren. Malar J. 2006;5:99. [DOI] [PMC free article] [PubMed]
- 44.Righetti AA, Adiossan LG, Ouattara M, Glinz D, Hurrell RF, N'Goran EK, et al. Dynamics of anemia in relation to parasitic infections, micronutrient status, and increasing age in south-central Côte d'Ivoire. J Infect Dis. 2013;207:1604–15. doi: 10.1093/infdis/jit066. [DOI] [PubMed] [Google Scholar]
- 45.Muller O, Traore C, Jahn A, Becher H. Severe anaemia in west African children: malaria or malnutrition? Lancet. 2003;361:86–7. doi: 10.1016/S0140-6736(03)12154-X. [DOI] [PubMed] [Google Scholar]
- 46.Mikkelsen L, Phillips DE, AbouZahr C, Setel PW, de Savigny D, Lozano R, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 47.Knopp S, Speich B, Hattendorf J, Rinaldi L, Mohammed KA, Khamis IS, et al. Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. PLoS Negl Trop Dis. 2011;5:e1036. [DOI] [PMC free article] [PubMed]
- 48.Sayasone S, Utzinger J, Akkhavong K, Odermatt P. Repeated stool sampling and use of multiple techniques enhance the sensitivity of helminth diagnosis: a cross-sectional survey in southern Lao People's Democratic Republic. Acta Trop. 2015;141:315–21. doi: 10.1016/j.actatropica.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Rutstein OS, Rojas G. Guide to DHS statistics. Calverton: ICF International; 2006.
- 50.Paciorek CJ, Stevens GA, Finucane MM, Ezzati M. Children's height and weight in rural and urban populations in low-income and middle-income countries: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e300–9. doi: 10.1016/S2214-109X(13)70109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vollmer S, Harttgen K, Subramanyam MA, Finlay J, Klasen S, Subramanian S. Association between economic growth and early childhood undernutrition: evidence from 121 Demographic and Health Surveys from 36 low-income and middle-income countries. Lancet Glob Health. 2014;2:e225–34. doi: 10.1016/S2214-109X(14)70025-7. [DOI] [PubMed] [Google Scholar]
- 52.Kaltenthaler EC, Drasar BS. The study of hygiene behaviour in Botswana: a combination of qualitative and quantitative methods. Trop Med Int Health. 1996;1:690–8. doi: 10.1111/j.1365-3156.1996.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 53.WHO. Gobal Targets 2025. To improve maternal, infant and young child nutrition. Geneva: World Health Organization; 2015. http://www.who.int/nutrition/global-target-2025/en/. Accessed 21 July 2016.
- 54.Sawyer SM, Afifi RA, Bearinger LH, Blakemore S-J, Dick B, Ezeh AC, et al. Adolescence: a foundation for future health. Lancet. 2012;379:1630–40. doi: 10.1016/S0140-6736(12)60072-5. [DOI] [PubMed] [Google Scholar]
- 55.FAO. WHO UNU. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. Rome: Food and Agriculture Organization, World Health Organization, United Nations University; 2001. [Google Scholar]
- 56.Pullan RL, Gitonga C, Mwandawiro C, Snow RW, Brooker SJ. Estimating the relative contribution of parasitic infections and nutrition for anaemia among school-aged children in Kenya: a subnational geostatistical analysis. BMJ Open. 2013;3:e001936. [DOI] [PMC free article] [PubMed]
- 57.Yap P, Utzinger J, Hattendorf J, Steinmann P. Influence of nutrition on infection and re-infection with soil-transmitted helminths: a systematic review. Parasit Vectors. 2014;7:229. doi: 10.1186/1756-3305-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoltzfus RJ. Defining iron-deficiency anemia in public health terms: a time for reflection. J Nutr. 2001;131:565S–7. doi: 10.1093/jn/131.2.565S. [DOI] [PubMed] [Google Scholar]
- 59.Belizario VY, Jr, Totanes FI, de Leon WU, Lumampao YF, Ciro RN. Soil-transmitted helminth and other intestinal parasitic infections among school children in indigenous people communities in Davao del Norte, Philippines. Acta Trop. 2011;120(Suppl 1):S12–8. doi: 10.1016/j.actatropica.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Belizario Jr VY, Liwanag HJ, Naig JR, Chua PL, Madamba MI, Dahildahil RO. Parasitological and nutritional status of school-age and preschool-age children in four villages in Southern Leyte, Philippines: lessons for monitoring the outcome of community-led total sanitation. Acta Trop. 2015;141:16–24. [DOI] [PubMed]
- 61.WHO, UNICEF, USAID . Improving nutrition outcomes with better water, sanitation and hygiene: practical solutions for policies and programmes. Geneva: World Health Organization; 2015. [Google Scholar]
- 62.Ministère de la Santé (Burkina Faso) Plan national de développement sanitaire 2011–2020. Ouagadougou: Ministère de la Santé; 2011. [Google Scholar]
- 63.Ministère de la Santé (Burkina Faso) Plan strategique nutrition 2010–2015. Ouagadougou: Ministère de la Santé; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article will not be shared. The paper is written as part of the academic degree of a PhD and therefore the data will be used exclusively by the author.


