Figure 2.
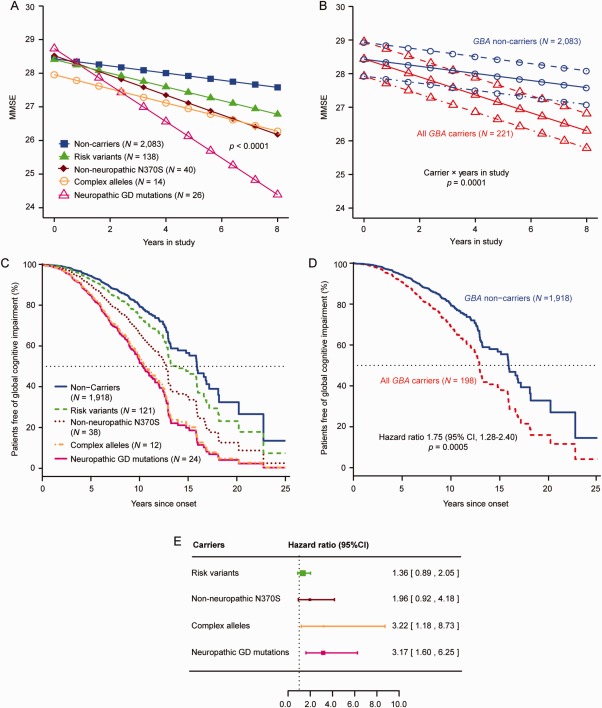
Specifically neuropathic GD mutations accelerate cognitive decline in patients with PD. (A,C,E) Neuropathic GD mutations carriers were linked to a more‐rapid cognitive decline in PD (in heterozygotes). (A) Neuropathic GD mutations predicted decline in Mini–Mental State Exam scores over time in the generalized longitudinal mixed model meta‐analysis in heterozygous patients. Illustrative mean scores on the MMSE across time predicted from the estimated fixed‐effect parameters in the mixed random‐ and fixed‐effects model analysis are shown for Parkinson's patients carrying specific types of GBA mutations and those without a GBA mutation. Carriers of a neuropathic GD mutation showed accelerated longitudinal cognitive decline compared to noncarriers with p < 0.0001, adjusting for the covariates of age at enrollment, sex, duration of PD upon enrollment, and years of education. Illustrative mean scores on the MMSE across time for PD patients with a neuropathic GD mutation are shown as magenta triangles; values for PD patients without a GBA mutation are represented as blue squares. Illustrative means scores on the MMSE across time for carriers of a risk variant (heterozygous carriers of E326K, T369M, and E388K; green triangles), the non‐neuropathic N370S mutation (brown rhombi), or complex GBA alleles (orange circles) are also shown. (B) Illustrative mean MMSE scores across time predicted from the estimated fixed‐effect parameters in the mixed random‐ and fixed‐effects model analysis are shown for Parkinson's patients without a GBA mutation (noncarriers) and those carrying any of the GBA mutations (all carriers). Carriers had overall a more‐rapid decline in cognitive function (as measured by serial MMSE) compared to noncarriers with p = 0.0001, after adjusting covariates (solid lines indicate mean value of disease duration at enrollment; dotted‐dashed lines indicate 1 SD longer disease duration at enrollment; and dashed lines indicate 1 SD shorter disease duration at enrollment). (C) Covariate adjusted survival curves for Parkinson's patients without a GBA mutation (noncarriers; blue line) and those carrying specific types of GBA mutations: risk variants (green, interrupted line), the common, non‐neuropathic N370S mutation (brown, dotted line), neuropathic GD mutations (magenta line), or complex GBA alleles (orange, dotted‐dashed line). (D) All carriers of a GBA mutation, taken together, had an overall hazard ratio for global cognitive impairment of 1.75 (95% CI, 1.28–2.40) compared to noncarriers with p = 0.0005, adjusting for age of onset, sex, years of education, and study. The covariate adjusted survival curves are shown (carriers, red interrupted line; noncarriers, blue line). The means of covariate‐adjusted predicted values are visualized. (E) The forest plot shows hazard ratios for global cognitive impairment in carriers of one of these specific types of GBA mutations. The hazard ratio for global cognitive impairment in carriers of neuropathic GD mutation was 3.17 (95% CI, 1.60–6.25; magenta). The squares represent point estimates, with the height of the square inversely proportional to the standard error of the estimates. The horizontal lines indicate 95% confidence intervals of the estimates. In (A), the group of patients with neuropathic GD mutations includes 26 heterozygous carriers with the following mutations: 12 with L444P, 2 with R463C, and 1 each of R257Q, 84dupG, R120W, D409H, R359X, P266L, N462K, A456P, L444R, G377S, H255Q, and G195E. The 14 carriers of complex alleles shown in (A) included 8 patients with E326K and D140H, 1 with E326K and T369M, 1 with E326K and R463C, and 1 with E326K and R257Q; and homozygous carriers of E326K/E326K, T369M/T369M, and E326/E326K/L444P/L444P genotypes, respectively. In (C), the group of patients with neuropathic GD mutations includes 24 heterozygous carriers with neuropathic GD mutations: 12 with L444P, 2 with R463C, and 1 each of R257Q, 84dupG, R120W, R359X, P266L, A456P, L444R, G377S, H255Q, and G195E. The 12 carriers of complex alleles shown in (C) included 6 patients with E326K plus D140H mutations, 1 with E326K plus T369M, 1 with E326K plus R463C, and 1 with E326K plus R257Q; and homozygotes carriers with E326K/E326K, T369M/T369M, and E326/E326K/L444P/L444P, respectively. It should be note that in the Cox proportional hazards analyses, the number of mutation carriers differs from that in the mixed fixed‐ and random‐effects analysis, attributed to the removal of subjects, who had already reached the endpoint at enrollment (left censored). The number of mutation carriers available for this analysis also differs from the number of carriers shown in Figure 1 because partially genotyped samples were here included, whereas data only for fully sequenced samples are shown in Figure 1. CI = confidence interval; GD = Gaucher's disease; SD = standard deviation.
