Abstract
Background
Little is known about whether hepatitis B surface antigen (HBsAg) seroconversion (SC) contributes to any survival benefits for patients with hepatocellular carcinoma (HCC).
Methods
All patients with hepatitis B-related HCC and HBsAg seroclearance between 1989 and 2013 were identified. Case- and control-groups were matched according to their stage of disease and mode of treatment. Baseline demographics, liver function, and overall survivals (OS) were compared between these two groups.
Results
Thirty-nine HCC cases with HBsAg SC were identified, and 312 non-seroconversion (NSC) HCC cases were matched. Forty-eight percent of patients had curative resections, 14% were treated with ablation and 38% were for palliation. Age of patients in SC group was older than those in NSC group (P=0.026). Although there was significantly better liver function in SC vs. NSC groups in terms of bilirubin (P=0.027), albumin (P=0.003), AST (P=0.001) and ALT (P<0.001), there was no overall difference in Child-Pugh grade among the two groups. In regarding tumour pathology, SC commonly presented with solitary tumour nodule as compared to multiple nodules in NSC (P=0.027), and was also frequently associated with a normal background liver parenchyma (P<0.001). Although no survival benefit was confirmed in log-rank analysis between SC and NSC, the absolute 5-year survival of SC group was better in resection (72.2% vs. 55.3%), ablation (83.3% vs. 57.4%) and palliation (24.4% vs. 14.4%).
Conclusions
HCC patients with HBsAg SC are associated with a better background liver parenchyma and function, and might contribute to an improved long-term survival.
Keywords: Hepatocellular carcinoma (HCC), hepatitis B, seroconversion (SC)
Introduction
Hepatocellular carcinoma (HCC) is one of the commonest cancers in Asia, where hepatitis B viral (HBV) infection is endemic. Majority of these patients suffer from chronic HBV, which is defined as the presence of hepatitis B surface antigen (HBsAg) for more than 6 months. The loss of HBsAg with the development of anti-HBsAg indicates a resolution of the viral infection. Up to approximately 90% of the patients suffered from acute HBV infection would be able to clear the virus (1). For those patients that did not develop anti-HBsAg, they will progress to chronic hepatitis B, which is associated with a significant risk of developing liver cirrhosis and HCC (1).
Less than 1% of patients, who have been in the inactive phase of chronic HBV for many years, may lose the presence of HBsAg and develop anti-HBsAg (2,3). With long-term follow-up of patients with HBsAg seroconversion (SC), there is evidence suggesting that the incidence of HCC in these patients is significantly lower than the non-seroconverter (4). However, there is currently no data available in literature regarding whether there is any clinical and survival benefits for patients, who already had developed HCC, but with HBsAg SC after the initial HCC treatment.
The aim of this study is to investigate any difference in histological and hepatic function of patients with HCC secondary to hepatitis B, and also those who also developed HBsAg SC thereafter. Our second aim is to compare the overall survival (OS) of HCC between these two groups of patients.
Methods
Patients with chronic hepatitis B SC were identified retrospectively from a prospectively maintained database between 1989 and 2010. A single research assistant recorded all their clinical data prospectively into a computerized database. Patients were excluded from the study if they had co-existing hepatitis C or human immunodeficiency virus infection. Using a ratio of 1:8 [SC vs. non-seroconversion (NSC)], The HCC patients with the HBsAg SC group were case-matched against the NSC group according to their stage (AJCC/UICC 5th edition staging system) of the disease and their initial first treatment modality—resection, ablative therapy and palliation.
Laboratory, diagnostic and histological evaluation
All patients had laboratory confirmation of their hepatitis B status at the point of diagnosis, and had had their HBsAg status re-checked after their first treatment and subsequent routine follow-up. HBsAg SC is defined as the persistent absence of serum HBsAg antigenemia for at least 12 months and until follow-up (3).
Hepatic function prior to the first treatment was assessed by Child-Pugh scoring (5), routine liver function test, serum platelet count and international normalised ratio. Preoperative alpha-feto-protein (AFP) level was performed prior intervention. Indocyanine green (ICG) clearance test was routinely performed for patient prior liver resection. Patient with ICG ≤14% at 15 minutes was considered safe for major hepatectomy in our practice (6).
Preoperative status of cirrhosis was determined on the basis of the findings of background liver biopsy and/or imaging findings complemented with clinical features of portal hypertension or encephalopathy (7,8).
Diagnosis of HCC was made by contrasted computer tomography (CT) or magnetic resonance imaging, without liver biopsy (7). Main portal vein invasion or distant metastasis was considered contraindication for surgical intervention. Imaging of all HCC patients were evaluated for resectability in a multi-disciplinary meeting.
For those patients with liver resections and/or combined open ablative therapy, histopathological parameters including macro- and micro-vascular invasions, resection margin, tumour differentiation and the background liver status were evaluated.
Postoperative follow-up
For patients with resection or ablative treatment modalities, they were follow-up within 1 month from discharge, and 3 monthly thereafter. Surveillance contrast CT scan was performed between 4 to 6 weeks after the initial treatment/operation, and was then performed 2–4 months thereafter. AFP was routinely performed during follow-up. Recurrence was confirmed by either imaging from CT, dual tracer (11C-acetate/18F-FDG) PET/CT (9), or histopathological diagnosis.
Statistical analysis
The primary endpoint is to investigate for any difference in histological and hepatic functional parameters between SC and NSC groups; and the secondary endpoint is to compare OS among these two groups of patients in different treatment modalities. OS was defined as survival from the first diagnosis of HCC till death.
Categorical data were presented as frequencies and proportions (%), and were analysed using Pearson’s chi-squared test or Fisher’s exact test. Medians (range) were used to describe continuous data, and Mann-Whitney test was applied for the comparison of the two groups of patients. The Kaplan-Meier method was used to assess the OS for all treatment groups, and the log-rank test was used to compare the survival difference.
Results
There were approximately 5,000 patients, who were diagnosed with HCC and were seen in our hospital, between 1989 and 2013. Thirty-nine patients infected with chronic hepatitis B and subsequent HBsAg SC were identified and included into this study. Seventeen, 6 and 16 patients of these patients had resections, open ablative procedures and palliation respectively. Three hundred and twelve patients were matched for the NSC group. One hundred and thirty-six, 48, and 128 patients had resections, open ablative procedures and palliation respectively in the corresponding NSC group.
The median age for HCC diagnosis in the SC group was older (60 vs. 57 years, P<0.03). Patients in the SC group were associated with better liver function in terms of bilirubin (11 vs. 14 years, P<0.03), aspartate aminotransferase (31.0 vs. 49.5 years, P=0.001), and alanine aminotransferase (31.0 vs. 44.5 years, P<0.001). Majority of them had solitary liver nodule as compared to multifocal liver nodules in the NSC group (P=0.039). There was no statistical difference in Child-Pugh score between these two groups of patients (P=0.436) (Table 1).
Table 1. Patient demographic and laboratory data.
| Variables | Seroconversion (n=39) | Non-seroconversion (n=312) | P value |
|---|---|---|---|
| Follow-up duration (month) | 15.4 (0.2–91.6) | 25.0 (0–276.6) | 0.154 |
| Age [median (range)] | 60 (36.0–84.0) | 57 (26.0–84.0) | 0.027 |
| Sex (male: female) | 36:3 | 256:56 | 0.106 |
| Serum bilirubin (mg/dL) | 11 (5.0–27.0) | 14 (3.0–109.0) | 0.027 |
| Albumin (g/L) | 42 (26.0–48.0) | 39 (24.0–50.0) | 0.003 |
| Haemoglobin (g/dL) | 13.8 (7.9–18.5) | 13.6 (7.6–18.2) | 0.421 |
| INR | 1 (0.9–1.2) | 1.1 (0.8–1.6) | 0.019 |
| Platelet count (×109/µL) | 180 (78.0–713.0) | 151 (22.0–621.0) | 0.051 |
| AFP (ng/mL) | 49 (2.0–285,244.0) | 135.5 (1.0–1,335,900.0) | 0.064 |
| AST (IU/L) | 31 (18.0–420.0) | 49.5 (18.0–444.0) | 0.001 |
| ALT (IU/L) | 31 (11.0–371.0) | 44.5 (13.0–325.0) | 0.0003 |
| Child-Pugh grade | 0.436 | ||
| A | 30 (81.1%) | 245 (79.0%) | |
| B | 7 (18.9%) | 52 (16.8%) | |
| C | 0 (0%) | 13 (4.2%) | |
| Size of tumour (cm) [median (range)] | 4.6 (1.1–20.0) | 5 (0.5–26.0) | 0.615 |
| No. of tumour nodules [solitary (%)] | 30 (76.9) | 185 (59.9) | 0.039 |
| Treatment | – | ||
| Resection | 17 (43.6%) | 136 (43.6%) | |
| Ablative Tx | 6 (15.4%) | 48 (15.4%) | |
| Palliative/no Tx | 16 (41.0%) | 128 (41.0%) | |
| Pattern of recurrence | 0.066 | ||
| No recurrence | 12 (70.6%) | 50 (36.8%) | |
| Intrahepatic recurrence | 2 (11.8%) | 39 (28.7%) | |
| Extrahepatic recurrence | 1 (5.9%) | 15 (11.0%) | |
| Both recurrence | 2 (11.8%) | 32 (23.5%) | |
| UICC 5th edition | – | ||
| Stage I | 5 (12.8%) | 40 (12.8%) | |
| Stage II | 11 (28.2%) | 88 (28.2%) | |
| Stage IIIA | 6 (15.4%) | 48 (15.4%) | |
| Stage IIIB | 1 (2.6%) | 8 (2.6%) | |
| Stage IVA | 12 (30.8%) | 96 (30.8%) | |
| Stage IVB | 4 (10.3%) | 32 (10.3%) |
AFP, alpha-feto-protein.
For those patients with hepatectomies, there were no differences in macro- and microvascular invasion, tumour differentiations, and positive margins between SC and NSC groups. However, more NSC patients suffered from an underlying liver cirrhosis (P<0.001) and were associated with tumour infiltration to adjacent organs (P=0.006) (Table 2).
Table 2. Tumour characteristic for resection group in HCC patients with/without seroconversion.
| Tumour characteristic for resection group | Seroconversion (n=17, %) | Non-seroconversion (n=136, %) | P value |
|---|---|---|---|
| Absence of invasion into major vein | 14 (82.4) | 114 (83.8) | 1.000 |
| Tumour differentiation | 0.334 | ||
| Well | 2 (11.8) | 29 (21.3) | |
| Moderate | 8 (47.1) | 75 (55.1) | |
| Poor | 7 (41.2) | 30 (22.1) | |
| Undifferentiated | 0 (0) | 2 (1.5) | |
| Presence of microvascular invasion | 12 (70.6) | 72 (52.9) | 0.168 |
| Positive resection margin | 1 (5.9) | 9 (6.6) | 1.000 |
| ICG-R15 (% of total) | 11.7 (5.3–45.7) | 13.5 (2.0–78.0) | 0.466 |
| Pathological liver status | <0.001 | ||
| Normal | 9 (52.9) | 6 (4.4) | |
| Chronic hepatitis | 2 (11.8) | 48 (35.3) | |
| Cirrhosis | 6 (35.3) | 82 (60.3) | |
| Absence of invasion of adjacent organs other than gallbladder | 13 (76.5) | 129 (94.9) | 0.006 |
There was a trend towards a better OS for the SC group in combined treatment modalities, resection alone, ablative therapies and palliation. However, there was no statistical difference when OS was compared between SC and NSC in any of these treatment modalities in log-rank analyses. For recurrent pattern, majority of the SC (>70%) did not develop any recurrent disease, as opposed to over 60% of patients in the NSC group during their follow-up period. In spite of this, statistical difference was not achieved between these two groups (P=0.067).
One-, 3- and 5-year OS for SC vs. NSC were 65.6% vs. 63.7%, 62.1% vs. 44.5%, and 55.9% vs. 38.7% respectively. After resection, 1-, 3- and 5- year OS were 81.4% vs. 78.7%, 73.3% vs. 61.9%, and 73.3% vs. 52.6%. Similar trends suggesting better OS in SC group were also observed in open ablation therapy and in the palliation, with a 5-year survival of 83.3% vs. 56.6% and 26.4% vs. 16.8% respectively (Figures 1,2,3,4).
Figure 1.
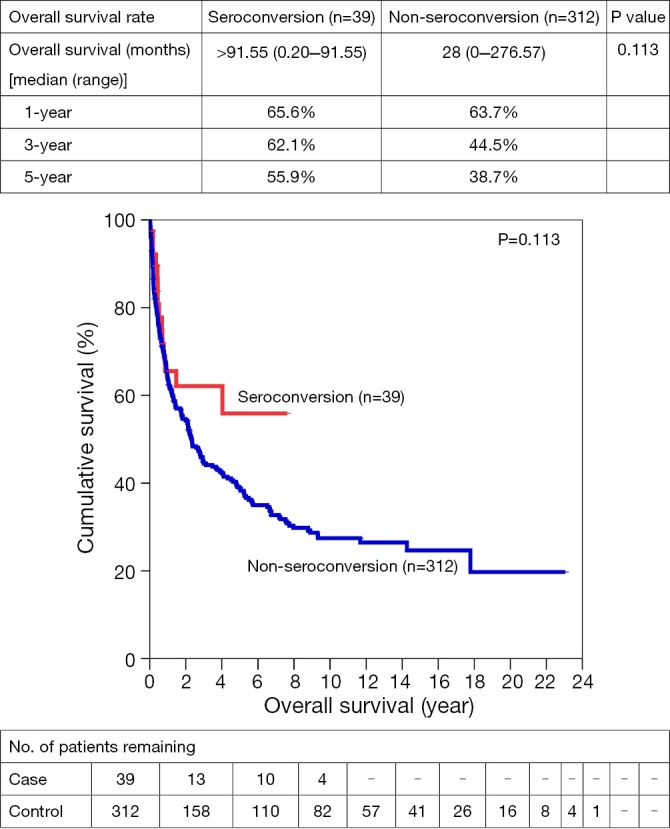
Overall survivals for all treatment groups for HCC patients with/without seroconversion.
Figure 2.
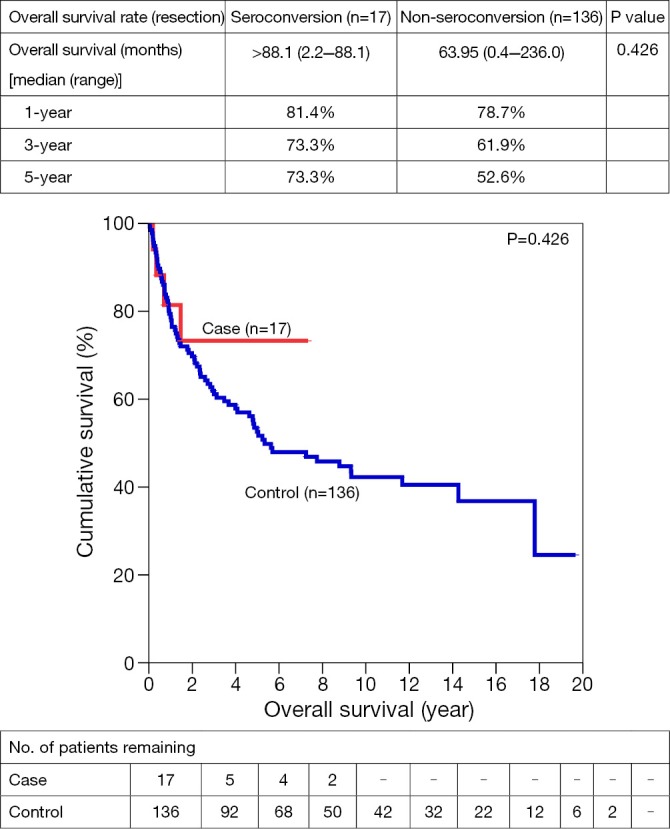
Overall survivals for hepatectomies in HCC patients with/without seroconversion.
Figure 3.
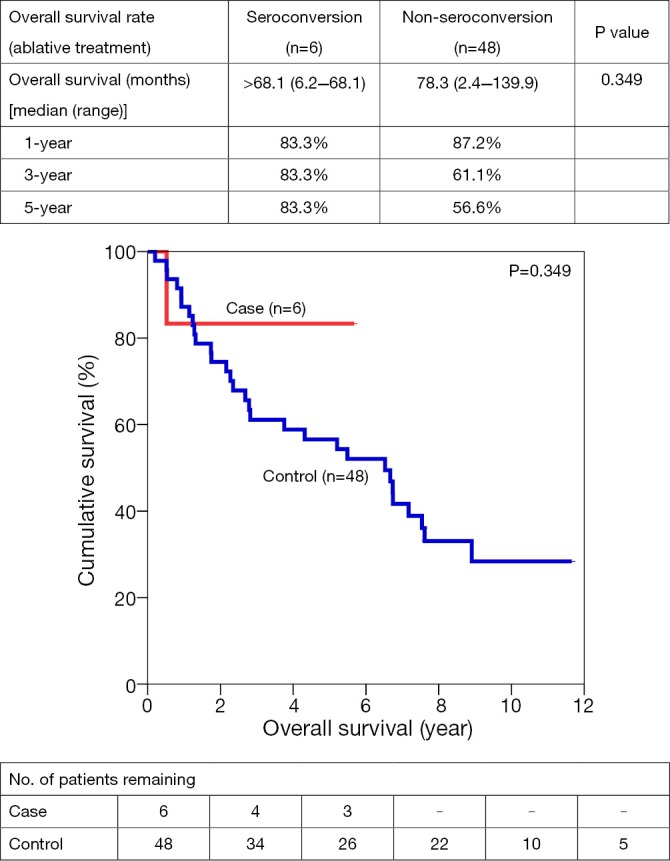
Overall survivals for ablative therapies in HCC patients with/without seroconversion.
Figure 4.
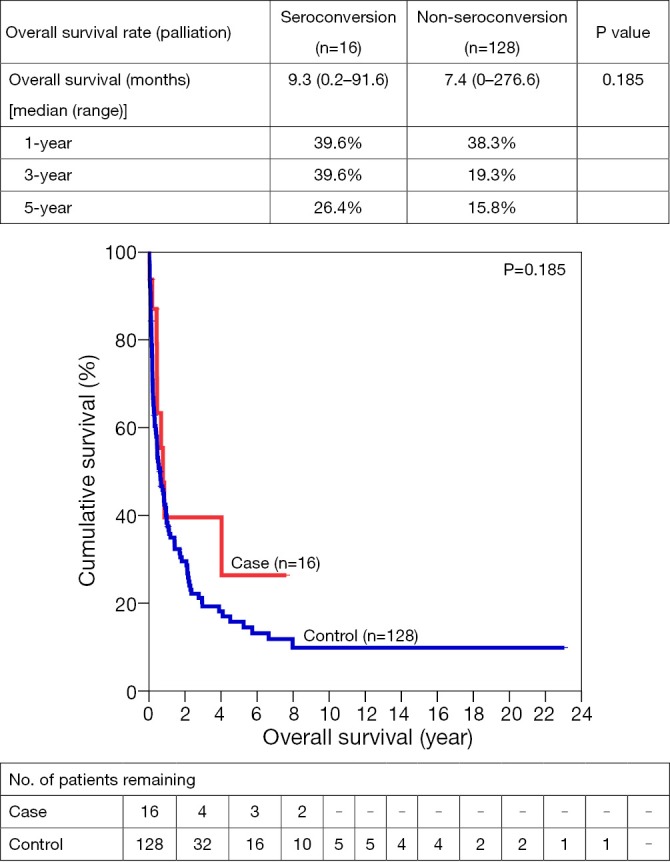
Overall survivals for palliation in HCC patients with/without seroconversion.
Discussion
This is the first and largest study comparing both clinical and survival outcomes of HCC patients with/without HBsAg SC. In this series, we have identified that HCC patient with SC is associated with better underlying liver function prior treatment, mainly solitary tumour nodule, and less underlying liver cirrhosis at surgical intervention. Although we did not demonstrate a significant survival benefits, there was a trend suggesting a better survival outcomes for HCC in patients with HBsAg SC.
In our cohort, we identified better preserved liver function in HCC patients of the SC group as compared to non-seroconverter. This finding is in consistent with other reports, which suggested significant reduction of alanine aminotransferase among patients with SC (3,10-12). This result can be explained by the fact that Hepatitis B virus is inactive among patients of the SC group, and therefore had less liver parenchymal damage. The other parameter that was different between our studied groups was age. In keeping with the findings in other study, older age group had been shown to be associated with an increased HBsAg SC (4). This makes sense because it is more likely that the older age group was at the inactive stage of the disease for a longer period than the younger group.
We have also identified an increased proportion of HCC patients without HBsAg seroclearance suffered from underlying liver cirrhosis or chronic inflammation. By sustained suppression of HBV DNA, achieving HBeAg and/or HBsAg SC, other studies have shown a reduction in fibrosis, as well as regression in cirrhosis (10,13,14). Along with this improved liver histology, it is logical to explain the improved liver function described above.
Comparing the histological characteristic for the resection groups of SC and NSC, it is interesting to note that the liver tumour in NSC group was associated with more adjacent organ involvement and an increased presence of multifocal tumour nodules in the resected specimen. The multifocal nature of HCC in the NSC group could be explained by the fact that majority of these patients suffered from some degree of liver fibrosis or cirrhosis, which is an intrinsic factor for the increased likelihood in developing multifocal tumour nodules.
Looking at the trends of the OS in resections, ablative therapy and palliation, they appeared to illustrate a survival difference. However, despite all these favourable biochemical and histological tumour characteristics after HBsAg SC, these had not truly been translated into survival benefits statistically. One of the main reasons for not achieving a statistical difference is likely due to the small number of SC patients in our cohort. In one of the largest studies over a 21-year period with a median follow-up of 62 months, only 2 out of 189 patients (1.1%), who had HBsAg SC, developed HCC (10). A similar incidence of HCC development in HBsAg seroconverters was also reported by Simonetti et al. (4). These studies highlighted the rarities of developing HCC in this cohort of patient, not alone the documentation of their long-term survival outcomes. Having identified a better absolute 5-year survival outcome in patients with HBsAg SC, we might be able to illustrate real survival benefits of SC with increasing the total number of patients over a longer period of time.
One of the limitations in this series is its retrospective study design. To maximise the validity of the data, all data was prospectively collected and recorded by a single data manager. Other major drawbacks are the incompleteness of this set of data in obtaining all viral antigens status including HBsAg positivity; the viral loads i.e., the amount of HBV DNA, and the length of anti-viral treatment before and after the HBsAg SC. This will provide a more meaningful comparison of HCC developed in our two studied groups.
In conclusion, this cohort is the first and largest study confirmed that HCC patients with HBsAg SC are associated with a better preserved underlying liver parenchyma and hepatic function, and might contribute to an improved long-term survival outcome.
Acknowledgements
None.
Ethical Statement: The study was approved by institutional ethic board and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology 2006;43:S173-81. 10.1002/hep.20956 [DOI] [PubMed] [Google Scholar]
- 2.Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology 2007;45:1187-92. 10.1002/hep.21612 [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Sheen IS, Chen TJ, et al. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology 1991;13:627-31. 10.1002/hep.1840130403 [DOI] [PubMed] [Google Scholar]
- 4.Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology 2010;51:1531-7. 10.1002/hep.23464 [DOI] [PubMed] [Google Scholar]
- 5.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-9. 10.1002/bjs.1800600817 [DOI] [PubMed] [Google Scholar]
- 6.Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg 1997;84:1255-9. 10.1002/bjs.1800840917 [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Tai DI, Chu CM, et al. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology 1988;8:493-6. 10.1002/hep.1840080310 [DOI] [PubMed] [Google Scholar]
- 8.Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol 1997;145:1039-47. 10.1093/oxfordjournals.aje.a009060 [DOI] [PubMed] [Google Scholar]
- 9.Cheung TT, Ho CL, Lo CM, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon's perspective. J Nucl Med 2013;54:192-200. 10.2967/jnumed.112.107516 [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Sheen IS, Chu CM, et al. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002;123:1084-9. 10.1053/gast.2002.36026 [DOI] [PubMed] [Google Scholar]
- 11.Perrillo RP, Brunt EM. Hepatic histologic and immunohistochemical changes in chronic hepatitis B after prolonged clearance of hepatitis B e antigen and hepatitis B surface antigen. Ann Intern Med 1991;115:113-5. 10.7326/0003-4819-115-2-113 [DOI] [PubMed] [Google Scholar]
- 12.You H, Wu X, Ou X, et al. Two patterns of alanine aminotransferase increase to predict long-term viral response in chronic hepatitis B patients: virus- or host-induced? Antivir Ther 2011;16:299-307. 10.3851/IMP1758 [DOI] [PubMed] [Google Scholar]
- 13.Chang TT, Lai CL, Kew Yoon S, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:422-30. 10.1002/hep.23327 [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Jia J. Why do I treat HBeAg-positive chronic hepatitis B patients with a nucleoside analogue. Liver Int 2013;33 Suppl 1:133-6. 10.1111/liv.12065 [DOI] [PubMed] [Google Scholar]


