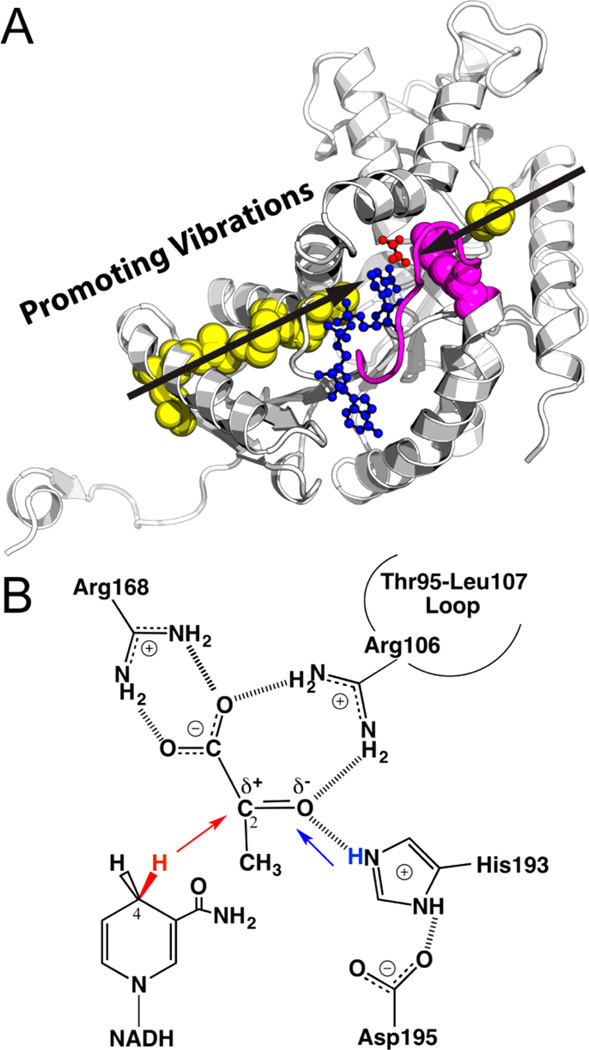
Figure 1.
(A) Active site and promoting vibrations of human heart LDH (one monomer from PDB code 1I0Z). The Thr95-Leu107 loop (magenta) encloses the active site and brings Arg106 (magenta sphere) into hydrogen bond contact with ligands to assist the hydride–proton transfer (illustrated in panel B). Transition path sampling simulations proposed LDH employs promoting vibrations (black arrows) to catalyze the hydride–proton transfer reaction. The distances between the hydride donor (NADH in blue) and acceptor (substrate analogue oxamate in red) are compressed by stochastic promoting vibrations that span the protein architecture (involving residues shown as spheres). (B) The chemical reaction catalyzed by LDH involves a hydride (red) transfer from NADD to the carbonyl carbon of pyruvate and a proton (blue) transfer from a protonated, conserved His residue to the carbonyl oxygen of pyruvate. The present study provides experimental evidence supporting concerted hydride–proton transfer mechanism in human heart LDH, and illustrates mass-dependent barrier crossing, consistent with the presence of promoting vibrations in LDH catalysis.