Abstract
Metastasis, a life-threatening complication of cancer, leads to the majority of cases of cancer-associated mortality. Unfortunately, the underlying molecular and cellular mechanisms of cancer metastasis remain to be fully elucidated. C-type lectins are a large group of proteins, which share structurally homologous carbohydrate-recognition domains (CRDs) and possess diverse physiological functions, including inflammation and antimicrobial immunity. Accumulating evidence has demonstrated the contribution of C-type lectins in different steps of the metastatic spread of cancer. Notably, a substantial proportion of C-type lectins, including selectins, mannose receptor (MR) and liver and lymph node sinusoidal endothelial cell C-type lectin, are important molecular targets for the formation of metastases in vitro and in vivo. The present review summarizes what has been found regarding C-type lectins in the lymphatic and hematogenous metastasis of cancer. An improved understanding the role of C-type lectins in cancer metastasis provides a comprehensive perspective for further clarifying the molecular mechanisms of cancer metastasis and supports the development of novel C-type lectins-based therapies the for prevention of metastasis in certain types of cancer.
Keywords: C-type lectins, lectin, tumor, metastasis
1. Introduction
The process of cancer metastasis comprises a multitude of consecutive steps. Hematogenous and lymphatic metastases are common routes for the spread of cancer. Generally, hematogenous metastasis of cancer consists of multiple sequential steps (1) (Fig. 1). In detail, cancer cells first escape from a primary site and then infiltrate the vascular endothelium into the bloodstream, where they flow to secondary sites, extravasate via the vascular endothelium into secondary sites and ultimately establish metastatic lesions. Cancer cells can also enter the blood vessel indirectly through the lymphatic duct (2). Similar to hematogenous metastasis, the complete lymphatic metastasis of cancer cells occurs by penetrating into the lymphatic endothelium, circulating in the lymphatic duct and subsequently metastasizing to distant lymph nodes (3). It is well recognized that the hematogenous metastasis of tumors is supported by the interactions between platelets, leukocytes and activated vascular endothelium. Aggregated platelets and leukocytes around tumor cells can defend tumor cells from immune elimination and promote subsequent dissemination. The activated endothelial cells can then mediate the adhesion of cancer emboli and the following extravasation (4). Selectins, commonly expressed on platelets, leukocytes and activated endothelium, are reported to be involved in the above steps via different roles (5). Mannose receptors (MRs), abundantly expression on afferent and efferent lymphatics, are involved in the lymphocyte homing and lymphatic metastasis of cancer cells by interacting with L-selectin. Notably, accumulating evidence indicates that adhesion molecules are associated with the biological behavior of primary cancer and the formation of secondary metastases (6,7). Dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), DC-SIGN-related (DC-SIGNR) and liver and lymph node sinusoidal endothelial cell C-type lectin (LSECtin) have been confirmed to show cell-adhesion functions, and have certain correlations with tumor metastasis (8–10). This introduces a novel concept of C-type lectins facilitating tumor metastasis. C-type lectins are a large group of proteins consisting predominantly of selectins, MR family members, Type II natural killer (NK) cell receptors and the DC-SIGN family. The majority of these are transmembrane proteins, whereas certain others are secreted as soluble proteins. The CRD of C-type lectins is the primary binding domain to several carbohydrates. Abnormal glycosylation leading to the alteration of carbohydrate structures is a warning sign of malignant transformation. The elevated expression of carbohydrate structures, including sialylated Lewis-x (sLex) and sialylated Lewis-a (sLea), correlates with cancer progression (11). Generally, the recognition of these carbohydrate structures is performed by the CRD of C-type lectins. Therefore, it is meaningful to investigate the roles of C-type lectins in tumor metastasis.
Figure 1.
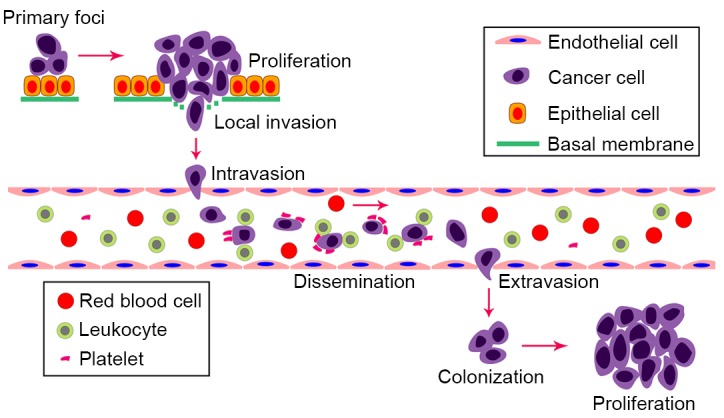
Successive steps of metastasis. The metastatic process begins with the proliferation of cancer cells in primary foci. The cancer cells escape from the primary site and intravasate the vascular endothelium into the bloodstream. Cancer cells interact with leukocytes and platelets protect them from immune elimination when circulating in the bloodstream. Subsequently, cancer cells are arrested by endothelial cells in the small capillaries of the secondary organ. Finally, cancer cells extravasate via the vascular endothelium into secondary sites and ultimately colonize metastatic lesions. C-type lectins are important in multiple metastatic steps.
2. CRD of C-type lectin
C-type lectins share a structurally homologous CRD, which bind to carbohydrate structures in a Ca2+-dependent manner. The CRD structure possesses a characteristic double-loop. The stability of this double-loop structure is dependent on two highly conserved disulfide bonds, and a set of conserved hydrophobic and polar interactions. The long loop domain is reported to function as a Ca2+-dependent carbohydrate-binding region (12). The carbohydrate-binding potential of C-type lectins is specifically supported by the CRD, and the adhesion affinity of different C-type lectins depends on the types of glycans. However, a number of CRD-containing proteins do not bind to carbohydrates. Generally, the subgroups of C-type lectins exhibit variation in their recognition of carbohydrates. The CRD of selectins recognizes sialylated, fucosylated glycoproteins, which frequently contain terminal tetrasaccharides sLex and sLea (13). MRs have two independent CRDs, which bind sulfated and mannosylated carbohydrates, respectively, and their recognitions is modulated by sialylation (14). Of note, the CRD of DC-SIGN recognizes glycoconjugates containing mannose, N-acetylglucosamine, fucose, and nonsialylated Lewisx and Ly glycans (15). Therefore, the CRDs of C-type lectins are associated with the recognition of several types of carbohydrate structures. Of note, the binding of C-type lectin CRDs to their carbohydrate ligands provides potential for the subsequent progression of cancer.
3. Selectins
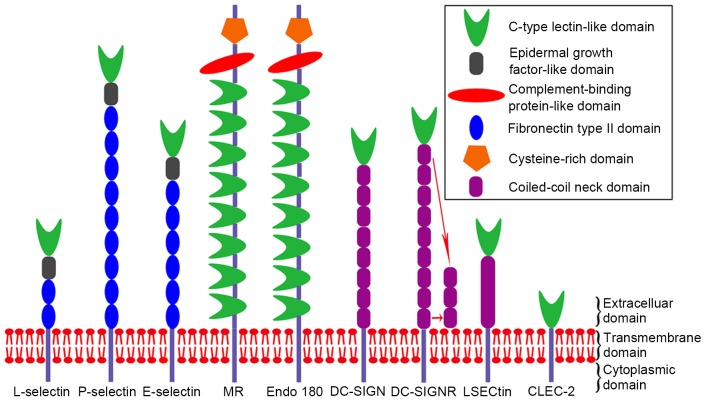
The selectin family comprises three members: L-, P- and E-selectin. All members of this family are type I transmembrane proteins with an N-terminal C-type lectin-like domain (CTLD), an epidermal growth factor-like domain, between two and nine complement-binding protein-like domains, a transmembrane domain, and a C-terminal cytoplasmic tail (Fig. 2). L-selectin is predominantly expressed by lymphocytes. E-selectin presents exclusively on the activated endothelium. P-selectin is expressed on activated platelets and endothelial cells (16). Selectins are a family of multifunctional adhesion molecules, which are important in physiological processes, including mediating the adhesion of leukocytes and platelets with the endothelium in the bloodstream. Analogously, another pathophysiological process, which is also associated with selectins, is the binding of circulating cancer cells to leukocytes and platelets, which defends them from immune elimination and facilitates metastatic spread. The N-terminal CTLD is the primary mediator for the interaction of selectins with their carbohydrate ligands. The malignant alteration comprises high affinity with abnormal glycosylation. Increased expression levels of sLex and sLea have been linked to the evolution and progression of several types of cancer (11). Of note, sLex and its isomer, sLea, tetrasaccharides are recognized by all selectins (13), and the selectin-based adhesion of sLex and sLea carbohydrates facilitates tumor metastasis.
Figure 2.
Structure of C-type lectins. All molecules are transmembrane proteins with cytoplasmic, transmembrane and extracellular domains. The distinction between these proteins is concentrated in the extracellular domain. The extracellular domain of selectins comprises a CTLD, an epidermal growth factor-like domain and between two and nine fibronectin type II domains. The extracellular domain of the MR family comprises eight CTLDs, a complement-binding protein-like domain and a cysteine-rich domain. The extracellular domain of the DC-SIGN family consists of a CTLD and between one and nine coiled-coil neck domains. The extracellular domain of CLEC-2 possesses only a CTLD. The CTLD is the primary domain involved in the recognition of carbohydrates on cancer cells. CTLD, C-type lectin-like domain; MR, mannose receptor; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; DC-SIGNR, DC-SIGN-related; LSECtin, liver and lymph node sinusoidal endothelial cell C-type lectin; CLEC-2, C-type lectin-like receptor 2.
4. L- and E-selectin in the lymphatic metastasis of cancer
The process of lymphocyte homing to peripheral lymph nodes requires the cooperation of adhesion molecules and chemokine receptors with their ligands (17). The molecular mechanism of cancer cell lymphatic metastasis is similar to that of lymphocyte homing (18). L-selectin is an adhesion molecule, which is essential in homing of lymphocytes to peripheral lymph nodes via interacting with a group of heterogenous glycoproteins, termed peripheral lymph node addressins (PNAds), expressed on high endothelial venules (HEVs) (19,20). The expression of MRs on lymphatic endothelium is identified as another ligand for L-selectin, which mediates the adhesion of lymphocytes to the lymphatic endothelium. MR is not expressed on HEVs, and PNAds are absent from the lymphatic endothelium (21). In vitro, silencing or inhibition of the expression of L-selectin on P388D1 macrophage-like lymphoid neoplasm cells significantly inhibits the adhesion of P388D1 cells to lymph nodes, and P388D1 cells transfected with L-selectin small interfering RNA, preincubated with MEL-4 or rat IgG have a significantly reduced metastatic rate to peripheral lymph nodes, compared with corresponding controls following footpad injections (22). P388D1 cells preincubated with heparin, an inhibitor for L-selectin, show similar results as those described above following footpad injections (23). In a transgenic mouse model expressing Tag (T), L-selectin (L), and Escherichia coli LacA (Z) in pancreatic β cells, the LTZ mice develop insulinomas, which specifically metastasize to lymph nodes, and the metastasis is inhibited by an anti-L-selectin monoclonal antibody in vivo (18). However, the adhesion of L-selectin expressed on a B-cell lymphoma cell line with lymph node HEVs in a L-selectin-dependent manner is not associated with increased incidence of lymphatic metastasis, which may be ascribed to the impaired function of L-selectin in partial tumor cells (24). Therefore, with the exception of regulating the trafficking of normal leukocytes, L-selectin can facilitate lymphatic metastasis of tumor cells (Fig. 3A and B). Currently, whether L-selectin promotes lymphatic metastasis via the same the process as the homing of lymphocytes via the ligand of PNAds or MR remains to be elucidated. E-selectin is expressed on activated endothelial cells and commonly promotes hematogenous metastasis. Of note, E-selectin and its carbohydrate ligand sLex are involved in the lymphatic metastasis of invasive breast micropapillary carcinoma (25).
Figure 3.
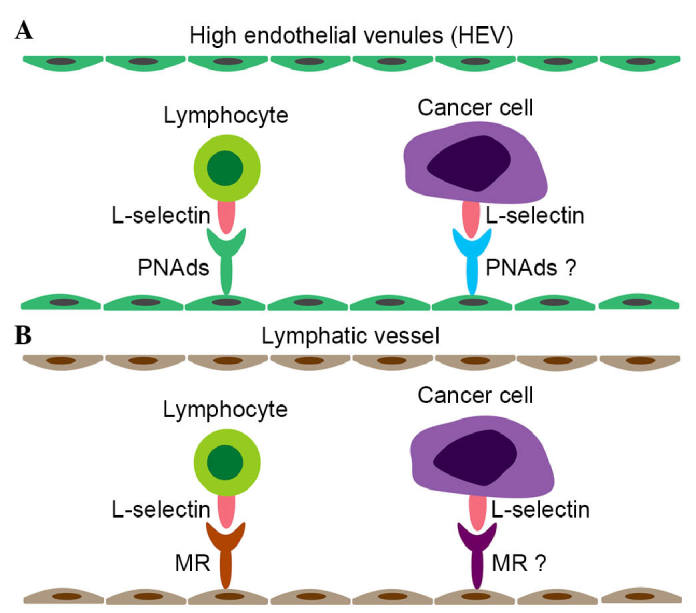
Roles of L-selectin are similar in lymphocyte homing and the lymphatic metastasis of cancer. The lymphatic metastasis of cancer has a high degree of similarity with lymphocyte homing. PNAds and MRs are ligands of L-selectin for lymphocyte adhesion to (A) HEVs and (B) lymphatic vessels, respectively. The attachment of cancer cells to HEVs and lymphatic vessels has been identified, although the corresponding ligands remain to be elucidated. PNads, peripheral lymph node addressins; HEV, high endothelial venule; MR, mannose receptor.
5. Selectins in the hematogenous metastasis of cancer
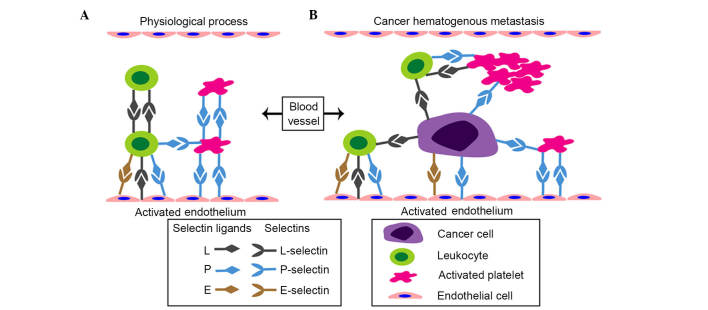
The interactions of cancer cells with platelets and leukocytes in the circulation, and the subsequent formation of cancer-cell-platelet-leukocyte emboli protect cancer cells from immune elimination, facilitate their adhesion to the endothelium and support the development of secondary metastastic foci (26). The selectins expressed on platelets, leukocytes and activated endothelial cells are crucial in establishing tumor cell thrombi and subsequent hematogenous metastasis (Fig. 4A and B). The expression of E-selectin on the cytokine-activated endothelium mediates the rolling of leukocytes, their subsequent arrest and their transmigration of the endothelium. Substantial evidence shows that E-selectin supports the attachment of tumor cells to the endothelium in a similar manner (5,27). The activation of E-selectin on the endothelium is cytokine-dependent, induced through the Ras/raf/mitogen-activated protein kinase pathway (28). Tumor cells metastasizing to the hepatic circulation can induce a cytokine cascade effect, resulting in the activation of E-selectin (29,30). Generally, colorectal cancer preferentially metastasizes to the liver and leads to a poor prognosis. E-selectin on activated hepatic sinusoidal endothelial cells interacts with carbohydrate ligands on colorectal cancer cells, including CD44 and hematopoietic cell E-/L-selectin ligand, mediating liver metastasis in vivo (28,31,32). This metastasis can be inhibited by E-selectin monoclonal antibody or C-raf antisense oligonucleotides, inhibiting the expression of E-selectin (29,33). In the metastasis of colon cancer to the lung, E-selectin is important in the formation of spontaneous metastasis in vivo (34). The E-selectin-CD44v4 interaction promotes the migration of breast cancer cells across the endothelium and transendothelial metastasis in vitro (35). The expression of gangliosides and Mac-2 on breast cancer cells are novel ligands for E-selectin, potentially mediating the formation of metastastic breast cancer (36,37). Furthermore, the interaction between bone-metastatic prostate cancer cells and the bone marrow endothelium in vitro is also E-selectin-dependent (38). Increased endothelial E-selectin can also facilitate the metastasis of pancreatic cancer cells to the liver in vivo (39). Interference of the cross-linking between sLe antigens with E-selectin indirectly suppresses the adhesion of tumor cells to the endothelium, inhibiting the formation of metastasis (40). Thus, it appears that E-selectin is a mediator for hematogenous metastasis in several types of cancer. Platelet-derived P-selectin promotes tumor metastasis by mediating the aggregation and adhesion of platelets to tumor cells, and the formation of platelet-cancer cell micro-emboli (26). Of note, P-selectin has been reported to bind to lymphoma, breast cancer, small cell lung carcinoma, colon cancer and neuroblastoma tumor cells, and these interactions are facilitated by multiple P-selectin ligands on the tumor cells, including P-selectin glycoprotein ligand-1, CD44, CD24, sulfatides and chondroitin sulfate glycosaminoglycans (41–46). In addition to its expression on platelets, P-selectin is present on endothelial cells and several types of tumor cell. The endothelial-derived P-selectin contributes to the adhesion of tumor cells to the microvasculature in a P-selectin-dependent manner (47). Similarly, P-selectin derived from tumor cells is an important metastatic target (47,48). Accumulating experimental evidence from the inhibition of P-selectin has elucidated the pivotal role of P-selectin in hematogenous metastasis. For example, the metastatic potential of gastric cancer in vivo is significantly attenuated by P-selectin monoclonal antibody (49). Heparin, an anti-metastatic reagent, suppresses P-selectin-mediated interactions of platelets to colon and non-small cell lung cancer cells (50,51). Semisynthetic sulfated tri mannose C-C-linked dimer, a P-selectin inhibitor, reduces the levels of metastasis in several animal models by suppressing P-selectin in vivo (52). L-selectin commonly supports the rolling of leukocytes along with endothelium and recruitment of leukocytes into inflammatory sites (53). Accordingly, the expression of L-selectin on neutrophils, monocytes and NK cells facilitates tumor progression and metastasis, supported by cancer cell-leukocyte-endothelial interactions (54,55). Podocalyxin-like protein on colon carcinoma cells and sialogucogylated podocalyxin on metastatic pancreatic cancer cells have been identified as functional L-selectin ligands, which are potentially associated with tumor metastasis (56). CD44, a transmembrane glycoprotein widely expressed on polytype tumor cells, possesses multiple variant isoforms and is identified as a facilitator for cancer metastasis (57). Each of the selectins attaches to variant isoforms of CD44 (CD44v) on colon carcinoma cells and promotes hematogenous metastasis in multiple organs (31,34,43,57,58). Therefore, the selectin-CD44v correlations may provide an explanation for the molecular mechanism underlying hematogenous metastasis in colon cancer. The adhesion of selectins with their ligands on tumor cells or hematocytes facilities the formation of tumor microemboli and prevents tumor cells from immune clearance, which finally mediates hematogenous transendothelial metastasis.
Figure 4.
Interactions between selectins and the physiological processes and hematogenous metastasis of cancer. (A) Physiological functions of selectins in the blood. The attachment of leukocytes and platelets to the activated endothelium are mediated cooperatively by three types of selectins. (B) Potential selectin-cancer cell correlations during the process of hematogenous metastasis. L- and P-selectins mediate the recruitment of leukocytes and platelets to cancer cells, and subsequently facilitate the formation of cancer-cell-platelet-leukocyte emboli, which protect cancer cells from immune elimination. E- and P-selectins are then critical in the attachment of cancer cells to the activated endothelium and in supporting metastatic spread.
6. Members of the MR family
The MR family includes MR, M-type phospholipase A2 receptor, DEC-205 and Endo180. All members of this family are type I transmembrane receptors, which consist of an N-terminal cysteine-rich domain, a single fibronectin type II domain, and 8–10 CTLDs (Fig. 2). MRs are expressed by selected populations of macrophages, DCs and nonvascular endothelium. Commonly, MRs mediate the phagocytosis of pathogens with expression of mannose, glucose, N-acetylglucosamine and fucose on the surface (14). In addition, MRs are abundant on afferent and efferent lymphatics. The presence of MRs on the lymphatic endothelium mediates the binding of lymphocytes to lymphatic vessels via its interaction with L-selectin on the surface of lymphocytes (21). Furthermore, the expression of MRs on lymphatic endothelium cells is involved in cancer cell adhesion to the lymphatic endothelium, which may contribute to the behavior of lymphatic metastasis. Endo180, an endocytic receptor predominantly expressed on stromal cells, is crucial in intracellular collagen degradation (59). The degradation is indicated by the interactions of Endo180 with the type I collagen and glycosylated collagens, which are from the fibronectin type II domain and CTLD, respectively (60). Similar to MR, the CRD of Endo180 also possesses a carbohydrate recognition function. However, Endo180 only exhibits binding potential to N-acetylglucosamine.
7. MR in the lymphatic metastasis of cancer
The expression of MR on lymphatic endothelial cells is involved in the adhesion of cancer cells to the lymphatic endothelium, which is recognized as a critical step for lymphatic spread. MR directs the adhesion of head, neck and breast cancer cells to the lymphatic endothelium in vitro (61). A tumor model of MR (−/−) mice following subcutaneous footpad injections of cancer cells exhibited reduced regional lymph node metastases, with significantly larger primary tumors. The attachment of lymphocytes and tumor cells to lymphatic vessels was a simultaneous reduction in this model (62). MR and CD44 have been identified as a receptor-ligand pair in supporting the migration of lymphocytes via the lymphatic vessel (63). The MR-CD44 interaction is dependent on the cysteine-rich domain of MR and chondroitin sulfate side chains of CD44. Generally, there is a level of similarity between lymphatic metastasis and the homing of lymphocytes to lymph nodes. As CD44 is present in the majority of malignancies, including breast cancer (64), colon cancer (65) and lung cancer (66), the correlation between the expression of CD44 on cancer cells with the MRs expressed on lymphatic endothelium may be associated with the lymphatic metastasis of these malignancies.
8. Role of Endo180 in tumor metastasis
Endo180, also known as urokinase plasminogen activator-associated protein, is the fourth member of the MR family and is predominately expressed on fibroblasts, endothelial cells and macrophages (67). Additionally, Endo180 is present on a variety of tumors, including those in prostate cancer (68), head and neck cancer (69), breast cancer (70) and glioma (71). Of note, the expression of Endo180 in epithelial cancer is frequently restricted to the stromal compartment, rather than the cancer cells themselves (72). Generally, degradation of the extracellular matrix is responsible for tissue remodeling, tumor invasion and metastasis. Endo180 functions as an endocytic receptor, is critical in the uptake and degradation of intracellular collagen, and also promotes extracellular matrix degradation (73). This degradation is predominantly mediated by the CRD of Endo180 with corresponding carbohydrate ligands. Currently, Endo180 is identified as a facilitator for the invasion and metastasis of multiple types of tumor. Endo180 is significantly associated with the overespression of membrane type 1-matrix metalloproteinase 14 in prostate cancer, which is associated with increased migration and metastasis in vitro (68). During the progression of head and neck squamous cell carcinoma, Endo180 is involved in the destruction of connective tissue through mediating cellular uptake and the degradation of collagen (69). In breast cancer, Endo180 has been confirmed to promote tumor growth in vivo (70). Notably, patients with breast cancer with elevated serum levels of Endo180 exhibit increased metastatic potential (74). Furthermore, a high expression level of Endo180 in glioma cells facilitates glioma invasion via collagen-containing matrices in an Endo180-dependent manner (71). Consistently, the downregulation of Endo180 is associated with decreased invasion and migration of glioma cells in vitro (75). These data demonstrated the specific relativity of Endo180 with the metastatic spread of tumors, and support that it may serve as a novel target for antitumor metastasis in a distinctive manner.
9. Type II NK cell receptor family in tumor metastasis
The NK-cell receptor family comprises several members. C-type lectin-like receptor 2 (CLEC-2) and lectin-like ox-low-density lipoprotein receptor-1 (LOX-1) are two representative molecules, which promote tumor metastasis. Notably, the extracellular domain of CLEC-2 possesses a CTLD (Fig. 2). CLEC-2 is expressed in abundance on platelets and acts as a platelet activation receptor. Platelets are crucial in physiological hemostasis, pathological bleeding and thrombosis within the bloodstream (76). Platelets can also protect tumor cells from immune elimination by facilitating the formation of platelet-tumor cell emboli, promoting the arrest of tumor cells at the endothelium and eventually establishing metastatic lesions. Podoplanin, functioning as an activating ligand for CLEC-2, is frequently upregulated in several types of tumor, which increases their invasive potential and mediates the hematogenous metastasis of tumors (77,78). Notably, the interaction between cancer cell-derived podoplanin and CLEC-2 on platelets is identified as a facilitator for metastatic spread (79). Takagi et al (77) reported that the anti-human podoplanin antibody, MS-1, can inhibit the CLEC-2-podoplanin interaction and subsequent platelet aggregation, and eventually suppress metastasis following xenografting of podoplanin-positive lung squamous cell carcinoma into NOD-SCID mice (77). Therefore, interference of the podoplanin-CLEC-2 attachment, which interferes with the platelet-tumor interaction, may be a molecular therapeutic route for hematogenous metastasis. Podoplanin is overexpressed in several types of cancer, including squamous cell carcinoma of the oral cavity, larynx and lung (80–82), malignant mesothelioma (83), colorectal adenocarcinoma (84) and brain tumors (85). Therefore, inhibiting podoplanin-CLEC-2 binding may be a promising strategy for attenuating the hematogenous metastasis of these types of cancer. LOX-1 is expressed primarily on the surface of endothelial cells and is key to the development of atherosclerosis. It is widely known that attachment of cancer cells to the endothelium and subsequent transendothelial migration are essential for metastasis. The upregulation of LOX-1 on the surface of human lung microvascular endothelial cells induced by tumor necrosis factor-α facilitates the adhesion and transendothelial migration of breast cancer cells in vitro (86). In this process, LOX-1 exhibits functional similarity to endothelial E-selectin in mediating tumor metastasis. Therefore, LOX-1 may be an important mediator for tumor metastasis in addition to E-selectin.
10. DC-SIGN family in tumor metastasis
The DC-SIGN family contains DC-SIGN, DC-SIGNR, LSECtin and CD23. All members of this family are type II transmembrane receptors, comprising a short NH2-terminal cytoplasmic tail, a transmembrane domain, an extracellular neck domain and a C-terminal CRD (Fig. 2). DC-SIGN is predominantly expressed on DCs, whereas DC-SIGNR and LSECtin are co-expressed by liver and lymph node sinusoidal endothelial cells (9,87). DC-SIGN, DC-SIGNR and LSECtin share common functions in that they serve as adhesion molecules and are involved in antipathogenic microorganism immunity (87). It is understood that DCs are essential for the antitumor immune response. The expression of DC-SIGN on DCs binds to Lewisx and Lewisy carbohydrates on tumor-associated carcinoembryonic antigens of colon cancer cells, suppressing the function of antitumor immunity of dendritic cells and subsequently inducing immune evasion, which is in favor of the progression and metastasis of colon cancer cells (8,88). Similar to DC-SIGN, the expression of LSECtin on B16 melanoma cells facilitates tumor progression by attenuating tumor-spectific T-cell responses (89). Therefore, one mechanism of DC-SIGN and LSECt in promoting tumor progression and metastasis may be through inhibiting the antitumor immune response. The association between DC-SIGN and LSECtin and tumor metastasis has been further demonstrated. Jiang et al (9) reported high expression levels of DC-SIGN in colon cancer tissues, which correlated positively with stage III–IV disease (9). Thus, DC-SIGN is associated with the metastatic potential of colon cancer cells, as lymphatic metastasis or distant organ metastasis are frequently present are in stages III–IV of disease. The expression of LSECtin on liver sinusoidal endothelial cells mediates the migration and metastasis of colon cancer cells to the liver. Consistently, the metastatic rate is markedly reduced in LSECtin-knockout mice following injections of colon cancer cells into the spleen in vivo (10). This finding may be valuable for elucidating the molecular mechanism of hepatic metastasis in colon cancer. Although the association between DC-SIGNR and tumor metastasis remains to be elucidated, previous studies have reported certain correlations between DC-SIGNR and tumors (9,90). Thus, the DC-SIGN family may be promising targets for tumor metastasis.
11. Targets for antimetastatic therapy
As mentioned above, the original interactions of C-type lectins with their corresponding ligands are critical in the formation of metastases. Inhibiting the expression of C-type lectins or interference of the binding between C-type lectins and their ligands may offer potential in targeting therapy. Selectins, vital mediators of platelet-cancer cell-leukocyte-endothelium interactions and the formation of metastases, have been identified as important targets and several selectin inhibitors have been found to have antimetastatic effects. For example, inhibiting the expression of E-selectin by cimetidine inhibits the adhesion of cancer cells to the endothelium and subsequent metastasis (40). P-selectin is a target of sulfated hexasaccharides in attenuating metastasis (52), and heparin effectively prevents the metastasis of various types of tumors by inhibiting selectins (47,50). In terms of the MR family, MR-targeted vaccines offer for an attractive strategy in antitumor therapy by increasing immune defense (91). Although the feasibility requires confirmation by further clinical trials, several studies, including in vivo studies, have supported the potential value of C-type lectin-dependent anti-metastatic targeting therapy.
12. Conclusions and perspectives
The present review summarizes representative C-type lectins crucial in the lymphatic and hematogenous metastasis of tumors, which are frequently dependent on the interactions between C-type lectins and corresponding carbohydrate ligands on tumor cells. The process of the metastatic spread of cancer cells comprises a series of successive steps, and each step of the cascade is essential to the establishment of a metastatic focus. The majority of C-type lectins, including selectins, MR, LSECtin, facilitate metastasis predominantly through involvement in the circulating of tumor cells in the blood or lymphatic vessels, adhesion to endothelial cells and subsequent transendothelial migration. However, Endo180 is involved in the invasion of tumor cells into the surrounding stroma by facilitating the degradation of extracellular matrix. An improved understanding of the involvement of C-type lectins in the diverse steps of cancer metastasis can offer a comprehensive perspective for further clarifying the complicated molecular mechanisms of cancer metastasis. The present review describes the novel concept of the association between C-type lectins and tumor metastasis, which provides direction in identifying additional metastatic molecules of C-type lectins. Although several C-type lectins molecules have been identified as mediators for tumor metastasis, the corresponding ligands remain to be fully elucidated. Further investigations are warranted to investigate the indeterminate ligands of C-type lectins. The elucidation of the importance of C-type lectins in cancer metastasis may provide novel insights into C-type lectin-based anti-metastatic therapy.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 5.Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169–177. doi: 10.1016/j.semcancer.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Arabzadeh A, Chan C, Nouvion AL, Breton V, Benlolo S, DeMarte L, Turbide C, Brodt P, Ferri L, Beauchemin N. Host-related carcinoembryonic antigen cell adhesion molecule 1 promotes metastasis of colorectal cancer. Oncogene. 2013;32:849–860. doi: 10.1038/onc.2012.112. [DOI] [PubMed] [Google Scholar]
- 7.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Gisbergen KP, Aarnoudse CA, Meijer GA, Geijtenbeek TB, van Kooyk Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005;65:5935–5944. doi: 10.1158/0008-5472.CAN-04-4140. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Zhang C, Chen K, Chen Z, Sun Z, Zhang Z, Ding D, Ren S, Zuo Y. The clinical significance of DC-SIGN and DC-SIGNR, which are novel markers expressed in human colon cancer. PLoS One. 2014;9:e114748. doi: 10.1371/journal.pone.0114748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo Y, Ren S, Wang M, Liu B, Yang J, Kuai X, Lin C, Zhao D, Tang L, He F. Novel roles of liver sinusoidal endothelial cell lectin in colon carcinoma cell adhesion, migration and in vivo metastasis to the liver. Gut. 2013;62:1169–1178. doi: 10.1136/gutjnl-2011-300593. [DOI] [PubMed] [Google Scholar]
- 11.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 13.Kansas GS. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 14.Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 16.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pals ST, de Gorter DJ, Spaargaren M. Lymphoma dissemination: The other face of lymphocyte homing. Blood. 2007;110:3102–3111. doi: 10.1182/blood-2007-05-075176. [DOI] [PubMed] [Google Scholar]
- 18.Qian F, Hanahan D, Weissman IL. L-selectin can facilitate metastasis to lymph nodes in a transgenic mouse model of carcinogenesis. Proc Natl Acad Sci USA. 2001;98:3976–3981. doi: 10.1073/pnas.061633698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 20.Girard JP, Moussion C, Förster R. HEV, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 21.Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, Jalkanen S. Mannose receptor is a novel ligand for L-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033–1042. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo Y, Wei W, Liu C, Zhao L, Wang L, Zhang J. Silencing L-selectin expression by siRNA attenuated metastasis of murine lymphoid neoplasm cell P388D1 to peripheral lymph nodes. Leukemia. 2007;21:180–183. doi: 10.1038/sj.leu.2404469. [DOI] [PubMed] [Google Scholar]
- 23.Wei W, Zuo Y, Hu Y, Wang L, Jia L, Zhang J. Heparin inhibits P388D1 cells adherence and metastasis to peripheral lymph nodes in vitro and in vivo. Lymphology. 2009;42:10–18. [PubMed] [Google Scholar]
- 24.Aviram R, Raz N, Kukulansky T, Hollander N. Expression of L-selectin and efficient binding to high endothelial venules do not modulate the dissemination potential of murine B-cell lymphoma. Cancer Immunol Immunother. 2001;50:61–68. doi: 10.1007/PL00006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J, Cui L, Liu F, Fan Y, Lang R, Gu F, Guo X, Tang P, Fu L. E-selectin and Sialyl Lewis X expression is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Int J Surg Pathol. 2010;18:193–200. doi: 10.1177/1066896908320832. [DOI] [PubMed] [Google Scholar]
- 26.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gout S, Tremblay PL, Huot J. Selectins and selectin ligands in extravasation of cancer cells and organs electivity of metastasis. Clin Exp Metastasis. 2008;25:335–344. doi: 10.1007/s10585-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson J, Wang JL, Barsky SH, Lee MC, Bischoff J, Nguyen M. Human colon cancer cells express multiple glycoprotein ligands for E-selecin. Int J Oncol. 2000;16:347–353. doi: 10.3892/ijo.16.2.347. [DOI] [PubMed] [Google Scholar]
- 29.Khatib AM, Fallavollita L, Wancewicz EV, Monia BP, Brodt P. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 2002;62:5393–5398. [PubMed] [Google Scholar]
- 30.Khatib AM, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59:1356–1361. [PubMed] [Google Scholar]
- 31.Hanley WD, Burdick MM, Konstantopoulos K, Sackstein R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 2005;65:5812–5817. doi: 10.1158/0008-5472.CAN-04-4557. [DOI] [PubMed] [Google Scholar]
- 32.Burdick MM, Chu JT, Godar S, Sackstein R. HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem. 2006;281:13899–13905. doi: 10.1074/jbc.M513617200. [DOI] [PubMed] [Google Scholar]
- 33.Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71:612–619. doi: 10.1002/(SICI)1097-0215(19970516)71:4<612::AID-IJC17>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Köhler S, Ullrich S, Richter U, Schumacher U. E-/P-selectins and colon carcinoma metastasis: First in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br J Cancer. 2010;102:602–609. doi: 10.1038/sj.bjc.6605492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zen K, Liu DQ, Guo YL, Wang C, Shan J, Fang M, Zhang CY, Liu Y. CD44v4 is a major E-selectin ligand that mediates breast cancer cell transendothelial migration. PLoS One. 2008;3:e1826. doi: 10.1371/journal.pone.0001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirure VS, Henson KA, Schnaar RL, Nimrichter L, Burdick MM. Gangliosiedes expressed on breast cancer cells are E-selectin ligands. Biochem Biophys Res Commun. 2011;406:423–429. doi: 10.1016/j.bbrc.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 37.Shirure VS, Reynolds NM, Burdick MM. Mac-2 binding protein is a novel E-selectin ligand expressed by breast cancer cell. PLoS One. 2012;7:e44529. doi: 10.1371/journal.pone.0044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL. Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res. 2004;64:5261–5269. doi: 10.1158/0008-5472.CAN-04-0691. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimoto K, Tajima H, Ohta T, Okamoto K, Sakai S, Kinoshita J, Furukawa H, Makino I, Hayashi H, Nakamura K, et al. Increased E-selectin in hepatic ischemia-reperfusion injury mediates liver metastasis of pancreatic cancer. Oncol Rep. 2012;28:791–796. doi: 10.3892/or.2012.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression. Cancer Res. 2000;60:3978–3984. [PubMed] [Google Scholar]
- 41.Raes G, Ghassabeh GH, Brys L, Mpofu N, Verschueren H, Vanhecke D, De Baetselier P. The metastatic T-cell hybridoma antigen/P-selectin glycoprotein ligand 1 is required for hematogenous metastasis of lymphomas. Int J Cancer. 2007;121:2646–2652. doi: 10.1002/ijc.23067. [DOI] [PubMed] [Google Scholar]
- 42.Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89:3385–3395. [PubMed] [Google Scholar]
- 43.Alves CS, Burdick MM, Thomas SN, Pawar P, Konstantopoulos K. The dual role of CD44 as a functional P selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am J Physiol Cell Physiol. 2008;294:C907–C916. doi: 10.1152/ajpcell.00463.2007. [DOI] [PubMed] [Google Scholar]
- 44.Garcia J, Callewaert N, Borsig L. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology. 2007;17:185–196. doi: 10.1093/glycob/cwl059. [DOI] [PubMed] [Google Scholar]
- 45.Monzavi-Karbassi B, Stanley JS, Hennings L, Jousheghany F, Artaud C, Shaaf S, Kieber-Emmons T. Chondroitin sulfate glycosaminoglycans as major P-selectin ligands on metastatic breast cancer cell lines. Int J Cancer. 2007;120:1179–1191. doi: 10.1002/ijc.22424. [DOI] [PubMed] [Google Scholar]
- 46.Stone JP, Wagner DD. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest. 1993;92:804–813. doi: 10.1172/JCI116654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ludwig RJ, Boehme B, Podda M, Henschler R, Jager E, Tandi C, Boehncke WH, Zollner TM, Kaufmann R, Gille J. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res. 2004;64:2743–2750. doi: 10.1158/0008-5472.CAN-03-1054. [DOI] [PubMed] [Google Scholar]
- 48.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JL, Chen WX, Zhu JS, Chen NW, Zhou T, Yao M, Zhang DQ, Wu YL. Effect of P-selectin monoclonal antibody on metastasis of gastric cancer and immune function. World J Gastroenterol. 2003;9:1607–1610. doi: 10.3748/wjg.v9.i7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei M, Tai G, Gao Y, Li N, Huang B, Zhou Y, Hao S, Zeng X. Modified heparin inhibits P-selectin-mediated cell adhesion of human colon carcinoma cells to immobilized platelets under dynamic flow conditions. J Biol Chem. 2004;279:29202–29210. doi: 10.1074/jbc.M312951200. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y, Wei M, Zheng S, Ba X, Hao S, Zeng X. Chemically modified heparin inhibits the in vitro adhesion of nonsmall cell lung cancer cells to P-selectin. J Cancer Res Clin Oncol. 2006;132:257–264. doi: 10.1007/s00432-005-0061-9. [DOI] [PubMed] [Google Scholar]
- 52.Borsig L, Vlodavsky I, Ishai-Michaeli R, Torri G, Vismara E. Sulfated hexasaccharides attenuate metastasis by inhibition of P-selectin and heparanase. Neoplasia. 2011;13:445–452. doi: 10.1593/neo.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Läubli H, Stevenson JL, Varki A, Varki NM, Borsig L. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 2006;66:1536–1542. doi: 10.1158/0008-5472.CAN-05-3121. [DOI] [PubMed] [Google Scholar]
- 55.Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dallas MR, Chen SH, Streppel MM, Sharma S, Maitra A, Konstantopoulos K. Sialofucosylated podocalyxin is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells. Am J Physiol Cell Physiol. 2012;303:C616–C624. doi: 10.1152/ajpcell.00149.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, Konstantopoulos K. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J. 2006;20:337–339. doi: 10.1096/fj.05-4574fje. [DOI] [PubMed] [Google Scholar]
- 58.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem. 2008;283:15647–15655. doi: 10.1074/jbc.M800543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivera-Calzada A, Robertson D, MacFadyen JR, Boskovic J, Isacke CM, Llorca O. Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180. EMBO Rep. 2003;4:807–812. doi: 10.1038/sj.embor.embor898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jürgensen HJ, Madsen DH, Ingvarsen S, Melander MC, Gårdsvoll H, Patthy L, Engelholm LH, Behrendt N. A novel functional role of collagen glycosylation: Interaction with the endocytic receptor uPARAP/Endo180. J Biol Chem. 2011;286:32736–32748. doi: 10.1074/jbc.M111.266692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irjala H, Alanen K, Grénman R, Heikkilä P, Joensuu H, Jalkanen S. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003;63:4671–4676. [PubMed] [Google Scholar]
- 62.Marttila-Ichihara F, Turja R, Miiluniemi M, Karikoski M, Maksimow M, Niemelä J, Martinez-Pomares L, Salmi M, Jalkanen S. Macrophage mannose receptor on lymphatics controls cell trafficking. Blood. 2008;112:64–72. doi: 10.1182/blood-2007-10-118984. [DOI] [PubMed] [Google Scholar]
- 63.Salmi M, Karikoski M, Elima K, Rantakari P, Jalkanen S. CD44 binds to macrophage mannose receptor on lymphatic endothelium and supports lymphocyte migration via afferent lymphatics. Circ Res. 2013;112:1577–1582. doi: 10.1161/CIRCRESAHA.111.300476. [DOI] [PubMed] [Google Scholar]
- 64.Götte M, Yip GW. Heparanase, hyaluronan, and CD44 in cancers: A breast carcinoma perspective. Cancer Res. 2006;66:10233–10237. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- 65.Wielenga VJ, van der Neut R, Offerhaus GJ, Pals ST. CD44 glycoproteins in colorectal cancer: Expression, function, and prognostic value. Adv Cancer Res. 2000;77:169–187. doi: 10.1016/S0065-230X(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 66.Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y, Wong MP. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 2010;5:e14062. doi: 10.1371/journal.pone.0014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jürgensen HJ, Madsen DH, Ingvarsen S, Melander MC, Gårdsvoll H, Patthy L, Engelholm LH, Behrendt N. A novel functional role of collagen glycosylation: Interraction with the endocytic collagen receptor uPARAP/ENDO180. J Biol Chem. 2011;286:32736–32748. doi: 10.1074/jbc.M111.266692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kogianni G, Walker MM, Waxman J, Sturge J. Endo180 expression with cofunctional partners MT1-MMP and uPAR-uPA is correlated with prostate cancer progression. Eur J Cancer. 2009;45:685–693. doi: 10.1016/j.ejca.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 69.Sulek J, Wagenaar-Miller RA, Shireman J, Molinolo A, Madsen DH, Engelholm LH, Behrendt N, Bugge TH. Increased expression of the collagen internalization receptor uPARAP/Endo180 in the stroma of head and neck cancer. J Histochem Cytochem. 2007;55:347–353. doi: 10.1369/jhc.6A7133.2006. [DOI] [PubMed] [Google Scholar]
- 70.Wienke D, Davies GC, Johnson DA, Sturge J, Lambros MB, Savage K, Elsheikh SE, Green AR, Ellis IO, Robertson D, et al. The collagen receptor Endo180 (CD280) is expressed on basal-like breast tumor cells and promotes tumor growth in vivo. Cancer Res. 2007;67:10230–10240. doi: 10.1158/0008-5472.CAN-06-3496. [DOI] [PubMed] [Google Scholar]
- 71.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engelholm LH, Ingvarsen S, Jürgensen HJ, Hillig T, Madsen DH, Nielsen BS, Behrendt N. The collagen receptor uPARAP/Endo180. Front Biosci (Landmark Ed) 2009;14:2103–2114. doi: 10.2741/3365. [DOI] [PubMed] [Google Scholar]
- 73.Curino AC, Engelholm LH, Yamada SS, Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS, Bugge TH. Intracelluar collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol. 2005;169:977–985. doi: 10.1083/jcb.200411153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmieri C, Caley MP, Purshouse K, Fonseca AV, Rodriguez-Teja M, Kogianni G, Woodley L, Odendaal J, Elliott K, Waxman J, Sturge J. Endo180 modulation by bisphosphonates and diagnostic accuracy in metastatic breast cancer. Br J Cancer. 2013;108:163–169. doi: 10.1038/bjc.2012.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi S, Yamada-Okabe H, Hamada K, Ohta S, Kawase T, Yoshida K, Toda M. Downregulation of uPARAP mediates cytoskeletal rearrangements and decreases invasion and migration properties in glioma cells. J Neurooncol. 2011;103:267–276. doi: 10.1007/s11060-010-0398-z. [DOI] [PubMed] [Google Scholar]
- 76.George JN. Platelets. Lancet. 2000;355:1531–1539. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 77.Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, Hatake K, Fujita N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One. 2013;8:e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowe KL, Finney BA, Deppermann C, Hägerling R, Gazit SL, Frampton J, Buckley C, Camerer E, Nieswandt B, Kiefer F, Watson SP. Podoplanin and CLEC-2 drive cerebrovascular patterning and integrity during development. Blood. 2015;125:3769–3777. doi: 10.1182/blood-2014-09-603803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res. 2012;129:S30–S37. doi: 10.1016/S0049-3848(12)70013-0. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 80.Martín-Villar E, Scholl FG, Gamallo C, Yurrita MM, Muñoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 81.Rodrigo JP, Garcia-Carracedo D, González MV, Mancebo G, Fresno MF, García-Pedrero J. Podoplanin expression in the development and progression of laryngeal squamous cell carcinomas. Mol Cancer. 2010;9:48. doi: 10.1186/1476-4598-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo T, Osawa M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]
- 83.Kimura N, Kimura I. Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83–86. doi: 10.1111/j.1440-1827.2005.01791.x. [DOI] [PubMed] [Google Scholar]
- 84.Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, Tsuruo T. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 85.Mishima K, Kato Y, Kaneko MK, Nishikawa R, Hirose T, Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 86.Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Letters. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Zhang F, Ren S, Zuo Y. DC-SIGN, DC-SIGNR and LSECtin: C-Type Lectins for Infection. Int Rev Immunol. 2014;33:54–66. doi: 10.3109/08830185.2013.834897. [DOI] [PubMed] [Google Scholar]
- 88.Nonaka M, Ma BY, Murai R, Nakamura N, Baba M, Kawasaki N, Hodohara K, Asano S, Kawasaki T. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells. J Immunol. 2008;180:3347–3356. doi: 10.4049/jimmunol.180.5.3347. [DOI] [PubMed] [Google Scholar]
- 89.Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z, Du X, Tang L, He F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74:3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z, Chen K, Yan L, Yang Z, Zhu Z, Chen C, Zeng J, Wei W, Qi X, Ren S, Zuo Y. Low expression of dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin-related protein in non-Hodgkin lymphoma and significant correlations with lactic acid dehydrogenase and β2-microglobulin. Biochem Cell Biol. 2013;91:214–220. doi: 10.1139/bcb-2012-0110. [DOI] [PubMed] [Google Scholar]
- 91.Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opin Biol Ther. 2004;4:1953–1962. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]