Abstract
The latest probable scenario in vaccination strategies is to prime one live attenuated vaccine candidate followed by boost dose of second vaccine candidate. In the present study, we primed the mice with a recombinant Bacille Calmette-Guerin (BCG) comprising Ag85B and ESAT-6 followed by boost doses of Ag85B, ESAT-6 and Ag85B-ESAT-6 fusion protein in the DDA adjuvant, separately. After boost doses of 8 and 12 weeks, the levels of antigen-stimulated T cells secreting interferon (IFN)-γ, the content of the IFN-γ, tumor necrosis factor-α and interleukin-4 in the splenocytes in vitro culture supernatant, the antigen-specific immunoglobulin (Ig)G titer from mouse serum, IgG subclass and the population of antigen-specific CD4+ and CD8+ T cells were detected. The present study showed that recombinant BCG along with boost doses of Ag85B or ESAT-6 individually did not induce efficient T-helper (Th) 1-type immune response. On the other hand, recombinant BCG with boost doses of Ag85B-ESAT-6 fusion protein enhanced longer lasting predominant Th1 immune response. This result suggested that Ag85B might synergize with ESAT-6 protein in order to produce better as well as effective immune response. Thus, the present study concluded recombinant BCG with boost doses of Ag85B-ESAT-6 fusion protein could be a good strategy to improve the immune protective efficacy.
Keywords: mycobacterium tuberculosis, prime-boost, recombinant Bacille Calmette-Guerin, vaccine
Introduction
Tuberculosis (TB) is one of the leading infectious diseases worldwide. The latest surveillance data by the World Health Organization reveals that in 2006, there were 9.2 million new cases and 1.7 million mortalities from TB (1). HIV co-infection markedly increases the risk of developing active TB disease (2). Available antibiotic chemotherapy regimens are becoming less effective in the face of emerging multidrug-resistant M.tb strains (3). The most efficient way to control any infectious disease is through prevention by a potent vaccine.
Bacille Calmette-Guerin (BCG) is the only currently available vaccine against TB since being first introduced in 1921. This vaccine has effective protection among children, particularly against military and TB meningitis, but is ineffective in protecting against adult pulmonary disease, particularly in TB endemic regions (4). BCG vaccine has failed to control TB epidemic after it has been used for 80 years. Therefore, there is a need to develop better or improved TB vaccine as an alternative to BCG. Subunit, DNA and virus vector vaccines, auxotroph M.tbs and recombinant BCGs are the important novel vaccine design strategies.
An effective vaccination strategy is the one that has ability to elicit protective immune response (5). Important vaccination strategies involve a prime-boost vaccination strategy (encompasses the benefits of both types of candidates), a heterologous prime-boost regimen comprising a prime with a viable vaccine candidate superior to BCG and a boost with a subunit vaccine candidate is likely to produce the most promising combination (6,7). Heterologous prime-boost immunization regimes induce higher levels of cellular immunity than homologous boosting with the same vaccine (8). Recently, heterologous prime-boost strategies based on the combination of DNA and protein subunit vaccines, BCG, or live attenuated viruses have been developed to improve the efficacy of vaccination against TB (9).
Recombinant BCG co-expressing the Ag85B and ESAT-6 is regarded as one of the most promising candidate vaccines. Mice vaccinated with rBCG have been observed to be better protected against aerosol infection with virulent M.tb in comparison to BCG (10). In the present study, we developed an immunization strategy to prime recombinant BCG encoding Ag85B-ESAT-6 (abbreviated as rBCG as below) along with boost doses of Ag85B, ESAT-6 and Ag85B-ESAT-6 fusion protein. We found that rBCG with increased doses of Ag85B-ESAT-6 fusion protein induced efficient and long lasting T-helper (Th) 1 immune response in comparison to rBCG alone or boost dose with single protein (Ag85B or ESAT-6).
Materials and methods
BCG and rBCG
Mycobacterium bovis BCG obtained from Shanghai Biological Products Institute Co., Ltd., Shanghai, China, rBCG was constructed in our lab (11), coding sequences for Ag85B and ESAT-6 were amplified from the M.tb H37Rv genomics DNA. Ag85B and ESAT-6 coding regions were cloned into the mycobacteral-E.coli shuttle vector PMV261, in which gene expression is under the control of the strong M.bovis HSP60 promoter. BCG was grown in Middlebrook 7H9 Medium (Difco Laboratories; BD Biosciences, Detroit, MI, USA) supplemented with 0.5% glycerol, 0.05% Tween-80 and 10% ADC or on solid Middlebrook 7H11 Medium (Difco laboratories) supplemented with 0.5% glycerol and 10% ADC. When the rBCG was cultured, the antibiotic kanamycin was added to the same medium at a concentration of 25 µg/ml.
Ag85B, ESAT-6, Ag85B-ESAT-6 fusion protein and DDA adjuvant
The Ag85B, ESAT-6 and Ag85B-ESAT-6 fusion proteins were cloned and expressed as previously described (11–13). Recombinant plasmid pQE30-ESAT-6, pET28a-Ag85B, and pET28a-Ag85B-ESAT-6 separately carrying ESAT-6, Ag85B and Ag85B-ESAT-6 gene as N-terminal histidine tagged fusion were transformed into the host BL21 (DE3) strain of E.coli (Novagen, Madison, WI, USA). Then they were induced for expression by 1 mM Isopropyl β-D-1-thiogalactoside. Cells were lysed and the lysate was applied to affinity chromatography using the His-Bind column (Novagen) as the protocol. Endotoxin was measured using the commercially available Quantitative Chromogenic End-point Tachypleus Amebocyte Lysate reactivity endotoxin kit (Chinese Horseshoe Crab Reagent Manufactory Co., Ltd., Xiamen, China). DDA was mixed into sterile distilled water to a concentration of 2.5 mg/ml, heated to 80°C, cooled to 25°C before use and delivered at 250 µg/dose (14).
Animal vaccination
Five-week-old female C57BL/6 mice (SLACCAS, Shanghai, China) were used in the ABSL-2 animal facility at Second Military Medical University (Shanghai, China). Mice received free access to food and water throughout this study. All experiments were performed in accordance to the local ethics committee. C57BL/6 mice (n=12 per group) were immunized subcutaneously at the dosage 5×106 CFU of BCG or rBCG in 200 µl phosphate-buffered saline (PBS). After 4 weeks of the prime immunization, the C57BL/6 mice were immunized with 10 µg Ag85B+DDA, 10 µg ESAT-6+DDA or 10 µg Ag85B-ESAT-6+DDA separately in the same way. Mice were sacrificed via cervical dislocation to analyze the immune responses at 8 and 12 weeks after the protein immunization. As an additional control, mice of the other group were injected with 250 µg of DDA adjuvant only. The experiment was repeated twice. Six groups are described in Table I.
Table I.
Vaccination of the 6 groups.
| Group | Prime with | Increased with |
|---|---|---|
| PBS | PBS | PBS+DDA (250 µg) |
| BCG | BCG (5×106 CFU) | PBS+DDA (250 µg) |
| rBCG | rBCG: Ag85B-ESAT-6 (5×106 CFU) | PBS+DDA (250 µg) |
| rBCG/A | rBCG: Ag85B-ESAT-6 (5×106 CFU) | Ag85B (10 µg)+DDA (250 µg) |
| rBCG/E | rBCG: Ag85B-ESAT-6 (5×106 CFU) | ESAT-6 (10 µg)+DDA (250 µg) |
| rBCG/AE | rBCG: Ag85B-ESAT-6 (5×106 CFU) | Ag85B-ESAT-6 (10 µg)+DDA (250 µg) |
Twelve mice per group were immunized subcutaneously. The C57BL/6 mice were primed with BCG or rBCG at the dose of 5×106 CFU in 200 µl PBS. After 4 weeks of the prime immunization, the mice were immunized with Ag85B, ESAT-6 or Ag85B-ESAT-6 fusion protein separately in the same way. PBS, phosphate-buffered saline.
This study was approved by the Animal Ethics Committee of Fudan University Animal Center.
ELISPOT assay for interferon (IFN)-γ from spleen cell culture
Eight and twelve weeks after the boost vaccination, mice were sacrificed, respectively, and their spleens removed aseptically in RPMI-1640 medium containing 10% fetal calf serum, 2 mM glutamine, 50 µM β-mercaptoethanol, 100 µg/ml streptomycin and 100 U/ml penicillin. Spleens were gently ground through a 70 µm cell strainer, and then single-cell suspensions were prepared with Lympholyte-M density-gradient centrifugation (CedarLane Lab, Burlington, NC, USA) according to the manufacturer's instructions. We used the mouse IFN-γ ELISPOT kit (U-Cytech Biosciences, Utrecht, The Netherlands) for detection of IFN-γ levels. Analyses were conducted on the cells from 5 mice in each group. The cells were diluted to the wells of the ELISPOT plate at 5×105 cells per well in culture medium, as described above, containing purified protein derivatives (PPD) 5 µg/ml, Ag85B (5 µg/ml), ESAT-6 (5 µg/ml) or phytohemagglutinin, 2 µg/ml, as positive control as stimulus. The plate was incubated at 37°C, 5% CO2, 100% humidity for 36 h and detected the IFN-γ secreting T cells as the procedure. Spots were counted by use of an immunospot image analyzer. Wells with <5 spots were not used for calculations.
Enzyme-linked immunosorbent assay (ELISA) analysis for IFN-γ, tumor necrosis factor (TNF)-α and interleukin (IL)-4
Single-cell suspensions were obtained and dilution of the cells in 2 ml culture medium contain the same concentration of stimulus as described above in the 12-well plate at 1×106 cells per well. The plate was incubated at 37°C, 5% CO2, 100% humidity for 36 h. The suspensions were collected of the cell culture for ELISA to detect the level of the cytokines (IFN-γ, TNF-α and IL-4). The cell deposits were harvested to prepare for flow cytometry. We used the mouse IFN-γ ELISA, TNF-α ELISA and IL-4 ELISA kits (eBioscience, Inc., San Diego, CA, USA) for detection of IFN-γ, TNF-α and IL-4. The concentration of the cytokines (IFN-γ and TNF-α) in the suspension was calculated according to the standards curve.
ELISA analysis for immunoglobulin (Ig)G, IgG1, IgG2c
Sera were collected from the immunized animals to monitor the antibody response by ELISA. Corning Costar 9018 ELISA plates (Corning Costar, Inc., Corning, NY, USA) were coated with Ag85B (5 µg/ml) or ESAT-6 (5 µg/ml). The plates were blocked with PBS containing 1% bovine serum albumin (BSA) (Bovogen Biologicals PTY., Ltd., VIC, Australia). Sera were added at serial 2-fold dilution (beginning at a 1/100 dilution). After washing, and adding horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1 and IgG2c (SouthernBiotech, Birmingham, AL, USA) were diluted at 1/10,000, 1/1,000 and 1/1,000 separately in blocking buffer (PBS containing 1% BSA). Plates displayed color by o-phenylenediamine substrate. Antibody titers were expressed as reciprocal end point titers.
Flow cytometry analysis
Spleen tissue was obtained and prepared as single cell suspension as described above in cell staining buffer (BioLegend, Inc., San Diego, CA, USA). The debris was removed by filtration of the cell suspension through 70-µm nylon mesh strainer. Viable cells were counted and suspended in cell staining buffer at 1×107 cells/ml. Cell suspensions (100 µl) were distributed into aseptic Eppendorf plastic tubes. Cells were blocked with PBS containing 1% BSA. Isotype controls of fluorescein isothiocyanate (FITC) and phycoerythrin (PE) conjugated anti-mouse IgG2b were used, 0.25 µg FITC anti-mouse CD4 and 0.25 µg PE anti-mouse CD8 (eBioscience, Inc.) were added per million cells in a 100 µl total staining volume followed by incubation in the dark at 4°C for 20 min. Cell pellets were washed twice and resuspend in 0.5 ml of cell staining buffer for analyzing under the flow cytometer (FACSCalibur; BD Biosciences, Detroit, MI, USA), with appropriate machine settings. Ten thousand events were collected.
Data analysis
Statistical significance was determined using one-way ANOVA with Kruskal-Wallis tests and Dunnett tests of GraphPad Prism 5.0 for Windows (GraphPad Software, Inc., La Jolla, CA, USA). PBS group was regarded as negative control. The remaining 4 groups were compared with the rBCG group. P<0.05 was considered to indicate a statistically significant difference.
Results
Cytokine response
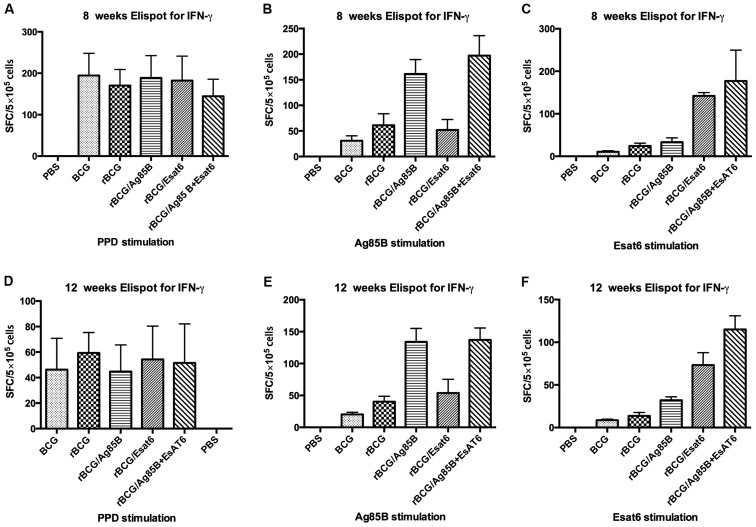
Single splenocyte suspensions from the 6 groups of mice after boost at 8 and 12 weeks were obtained and assayed for IFN-γ at 36 h post-stimulation with special antigen. An ELISPOT assay was used to determine the relative numbers of IFN-γ expressing cells in single splenocyte suspensions of mice immunized with different groups. The numbers of such cells were shown by spot-forming units (Fig. 1). Splenocytes from mice vaccinated with PBS as the negative control can hardly produce IFN-γ whether stimulated with PPD, Ag85B or ESAT-6. Splenocytes from mice vaccinated with all the groups except PBS produced IFN-γ at high level when stimulated with PPD at 8 weeks. They reduced from about 180 spot-forming cells (SFC) at 8 weeks to about 50 SFC at 12 weeks, but they showed no difference.
Figure 1.
The cellular immune response was measured in an ELISPOT assay with splenocytes isolated from C57BL/6 mice immunized with different groups at 8 weeks (A-C) and 12 weeks (D-F). Splenocytes were plated 5×105 cells per well in duplicate and incubated with PPD (5 µg/ml), Ag85B (5 µg/ml), ESAT-6 (5 µg/ml) for 36 h at 37°C, 5% CO2, 100% humidity for 36 h. Data are presented as means ± standard deviation. PPD, purified protein derivative.
When stimulated with Ag85B, the IFN-γ level of rBCG/A and rBCG/AE induced 3-4-fold higher than that of rBCG whether at 8 weeks or at 12 weeks. However, rBCG/E did not increase the IFN-γ level. Compared BCG with rBCG, rBCG induced higher IFN-γ level than BCG at 8 weeks, but at 12 week, they showed no difference. When stimulated with ESAT-6, rBCG/AE and rBCG/E increased >3-fold of rBCG at 8 weeks or 12 weeks. ESAT-6 is one of the RD1 genes and deleted from BCG vaccine, thus IFN-γ level in the BCG group can hardly be detected, whereas rBCG/E showed no difference with rBCG.
ELISA assay for IFN-γ and TNF-α in the splenocyte culture suspensions is shown in Table II. Stimulated with Ag85B, the IFN-γ level of the rBCG/A and rBCG/AE was nearly 9-fold higher than rBCG at 8 weeks. The IFN-γ level decreased at 12 weeks, but the significant difference was kept. rBCG/E and rBCG showed no difference of IFN-γ level at 8 and 12 weeks, respectively. When stimulated with ESAT-6, the IFN-γ level of rBCG/AE was approximately double that of rBCG/E, while they both much higher than rBCG at 8 weeks. rBCG/A induced higher a IFN-γ level than rBCG at 8 weeks. The IFN-γ level of all the groups decreased at 12 weeks, although the 3 groups were significant higher than rBCG. The limit of sensitivity of the kit was 4 pg/ml, and the IFN-γ level of the PBS and BCG was too low to be detected.
Table II.
Cytokine productiona (pg/ml).
| IFN-γ | TNF-α | |||
|---|---|---|---|---|
| Groups | 8 weeks | 12 weeks | 8 weeks | 12 weeks |
| Ag85B stimulus | ||||
| PBS | <4b | <4 | 78.3±10.9 | 60.4±7.1 |
| BCG | <4 | <4 | 123.2±11.1c,d | 92.7±16.9 |
| rBCG | 94.6±4.6 | 20.1±5.5 | 252.95±11.5 | 132.7±11.7 |
| rBCG/A | 909.2±116.7d | 262.8±49.6d | 239.1±21.5 | 191.0±26.0e |
| rBCG/E | 121.0±17.6 | 35.2±7.0 | 180.2±11.5d | 147.4±9.3 |
| rBCG/AE | 967.6±154.3d | 320.0±74.1d | 448.4±23.2d | 247.0±37.2d |
| ESAT-6 stimulus | ||||
| PBS | <4 | <4 | 18.5±3.6 | 16.2±3.4 |
| BCG | <4 | <4 | 104.7±11.8 | 95.9±11.3 |
| rBCG | 18.8±1.6 | <4 | 95.6±10.9 | 102.4±15.7 |
| rBCG/A | 83.6±5.5f | 27.1±10.2e | 148.3±11.2e | 173.4±23.4d |
| rBCG/E | 351.6±34.5d | 68.3±17.6d | 580.8±12.8d | 357.9±17.6d |
| rBCG/AE | 836.4±86.6d | 234.2±39.3d | 585.1±39.7d | 503.9±28.3d |
The content of IFN-γ and TNF-α was measured by ELISA assay in the suspensions of the splenocytes culture. Splenocytes were plated 1×106 cells per well duplicate and incubated with Ag85B (5 µg/ml), ESAT-6 (5 µg/ml) for 36 h at 37°C, 5% CO2, 100% humidity.
cytokine production by mouse spleen cells was assay by sandwich ELISA after stimulation with Ag85B.
the limit sensentivity of the kit was 4 pg/ml, and the level of production of IFN-γ was too low to be detected.
is highly significant as compared to rBCG group at the same time point and antigen stimulated (P<0.001)
is very significant as compared to rBCG group (0.001<P<0.01)
is significant as compared to rBCG (0.01<P<0.05). IFN, interferon; TNF, tumor necrosis factor; PBS, phosphate buffered saline.
When stimulated with Ag85B, rBCG/AE produced approximately 1.8-fold TNF-α level of rBCG at 8 and 12 weeks. rBCG/A showed no difference with rBCG at 8 weeks but a significantly higher rBCG at 12 weeks. rBCG/E and BCG was significantly lower than rBCG at 8 weeks, but they showed no difference at 12 weeks. When stimulated with ESAT-6, rBCG/AE and rBCG/E produced approximately 6-fold that of rBCG at 8 weeks. TNF-α level of rBCG/A was also higher than rBCG. At 12 weeks, the 3 groups (rBCG/AE, rBCG/A and rBCG/E) were significantly higher than rBCG.
The limit of sensitivity of the mouse IL-4 ELISA kit was 4 pg/ml, and the level of production of IL-4 was too low to be detected for all the groups. Thus, we did not include this in Table II.
Humoral response
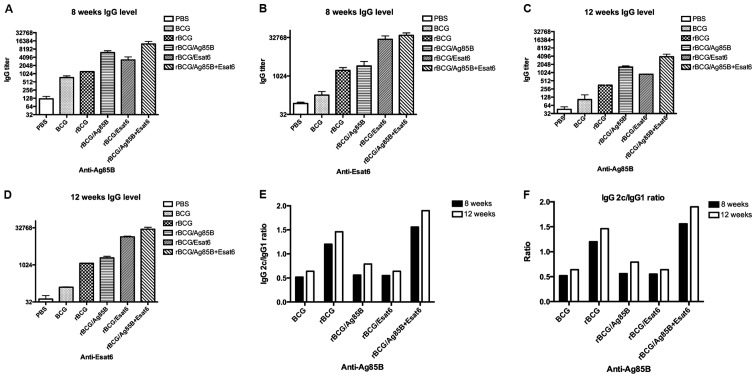
To evaluate humoral immune response after the vaccination, specific antibodies were determined in mice immunized with the antigen. Eight and 12 weeks following the last immunization, serum IgG1, IgG2c and total IgG antibody levels were measured by ELISA. Fig. 2A-D shows the relative concentrations of protein-specific total IgG antibodies in the sera of mice. Total IgG titers for Ag85B of the rBCG/A and rBCG/AE were higher than rBCG at 8 and 12 weeks. rBCG/E and BCG showed no difference with rBCG. Total IgG titers for ESAT-6 of the rBCG/E and rBCG/AE were enhanced >100-fold that of rBCG at 8 and 12 weeks. rBCG/A showed no difference with rBCG. The ESAT-6 gene was one of the RD1 regions of the BCG and did not exist in the BCG. Thus, we detected the ESAT-6 special antibody titer at a very low level.
Figure 2.
Antibody response against Ag85B (A and C) and ESAT-6 (B and D) in mice immunized in the six groups. Sera were collected from mice immunized as the six groups at 8 weeks and 12 weeks after boost vaccination, then examined for IgG (A-D) and the ratio of IgG2c/IgG1 (E and F). Data are presented as means ± standard deviation. Ig, immunoglobulin.
In C57BL/6 mice, the gene coding for IgG2a is deleted. Therefore, in the absence of a functional IgG2a gene, the IgG2c isotype was used as an indicator of a T-helper (Th)-type 1 response. The ratios of IgG2c/IgG1 were calculated to determine the induction of Th1 or Th2 responses in animals (Fig. 2E and F). As a result in response to Ag85B, the IgG2c/IgG1 ratios of the rBCG and rBCG/AE were higher than that of BCG, rBCG/A or rBCG/E and above to 1.2. At 12 weeks the IgG2c/IgG1 ratios of rBCG and rBCG/AE also kept higher than the other 3 groups.
In response to ESAT-6, the ratios of rBCG and rBCG/AE groups were higher than that of rBCG/A or rBCG/E. There were no obvious IgG1 and IgG2c titers detected in the mice immunized with BCG.
CD4+ T cell and CD8+T cell analyze
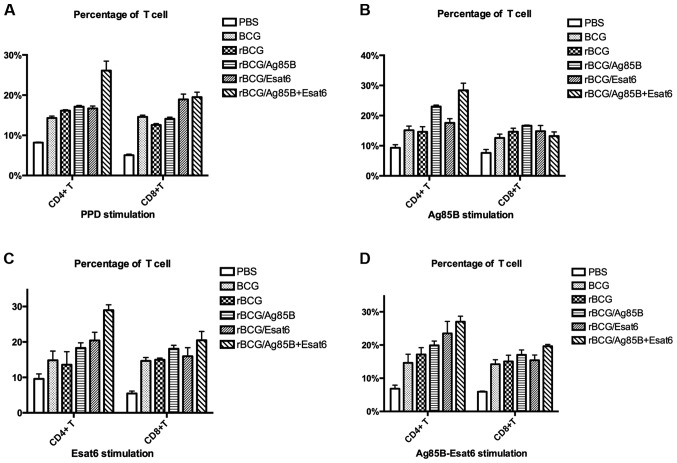
To investigate the alteration in the proportions of lymphoid cells in the spleen after vaccination, the T cells were stained with the cell surface markers for flow cytometry analysis. The CD4+ T-cell and CD8+ T-cell levels stimulated by different special antigen are shown in Fig. 3A-D.
Figure 3.
Analysis of T-cell percentage stimulated by PPD (A), Ag85B (B), ESAT-6 (C) and Ag85B-ESAT-6 (D). Splenocytes were isolated from mice at 8 weeks after boost vaccination and incubated with FITC-conjugated anti-CD4 and PE-conjugated anti-CD8. The percentages of CD4+ and CD8+ T cells were determined by flow cytometry. The results are expressed as the mean ± standard deviation.
The splenocytes were prepared from the mice at 8 weeks after the boost. When stimulated with PPD, rBCG/AE improved CD4+ T cells compared to rBCG. The CD8+ T-cell levels of the 4 groups (BCG, rBCG/A, rBCG/E and rBCG/AE) were higher than that of rBCG. Stimulated with Ag85B-ESAT-6, rBCG/E and rBCG/AE enhanced CD4+ T-cell level compared to rBCG. Only rBCG/AE enhanced CD8+ T-cell level as rBCG.
Discussion
Data from humans and several animal models have suggested that Th1 subset and IFN-γ are involved in the development of protective immunity against M.tb. Thus, increase in Th1 response or induction of higher levels of IFN-γ should lead to increased anti-mycobacterial activity (15,16). TNF-α has been shown to be critical for granuloma formation in mice (17), and in humans, targeted anti-TNF-α therapy for chronic inflammatory conditions could lead to reactivation of latent TB (18). IFN-γ and TNF-α contribute to the recruitment of monocytes and granulocytes (19) and activate the antimicrobial activity of macrophages (15). In the present study, we compared the immunogenicity of several heterologous prime-boost combinations based on rBCG and different protein subunit vaccines. rBCG showed higher IFN-γ and TNF-α levels than BCG at early time (<12 weeks after vaccination) as observed in our earlier study, but no statistical significant difference was observed after 12 weeks of vaccination. Additionally, the immunogenicity of rBCG gradually decreased with time. On the other hand, rBCG, along with boost doses of subunit protein vaccine kept levels of IFN-γ and TNF-α higher for a long period of time.
Vaccination with BCG or rBCG did not induce very high special antigen IgG titer at 12 weeks, but the boost with subunit vaccine, the special antigen IgG titer was expressed higher and sustained for a long time. rBCG/AE showed higher IgG titer than rBCG/A or rBCG/E for the same special antigen. This showed Ag85B has the ability to synergize with ESAT-6 in order to improve the IgG titer.
The ratios of IgG2c/IgG1 were calculated to determine the levels of induction of the Th1/Th2 responses in animals. The increasing ratio of IgG2c/IgG1 revealed the ability of induction of Th1 protection immune response. rBCG/AE showed the highest ratio of IgG2c/IgG1, followed by rBCG, and rBCG/E and rBCG/A, which were almost the same as BCG. This result showed Ag85B may have coordinated well with ESAT-6 to promote the immune response to Th1-type, while rBCG/A or rBCG/E could not improve the ratio of IgG2c/IgG1 ideally. The above observation could be justified by the fact that with rBCG with single protein, whether Ag85B or ESAT-6 may break the balance of the antigen-induced immune response; thus, failing to drive the immune response to the Th1-type. The result clearly showed that rBCG/AE facilitated the Th1 immune response as compared with BCG, rBCG, rBCG/A or rBCG/E.
Studies over the past few years of anti-TB immunity in mice concluded that immunity is mediated predominantly by CD4 Th1 cells with the aid of CD8 T cells. It is known that immune responses against M.tb are mediated by CD4+ T cells, although recent evidence indicates that CD8+ T cells also contribute to antimycobacterial immunity (15). CD8+ T cells appear to mediate immune surveillance of latent TB infection (20) and to be involved in macrophage activation (21). Using rBCG as the priming immunization in C57BL/6 mice and then boosting these mice with Ag85B-ESAT-6 fusion protein induced higher levels of both antigen specific CD4+ T and CD8+ T cells. rBCG/A or rBCG/E did not improve CD4+ T and CD8+ T cells significantly.
Ag85B and ESAT-6 are very promising vaccine candidate molecules for several reasons: i) They are strongly recognized by T-cell antigens in the first phase of infection (22,23); ii) they have demonstrated protective efficacy in animal models (10,24); and iii) they contained numerous well-characterized epitopes recognized in TB patients. Additionally, the present study showed that the boost with Ag85B-ESAT-6 fusion protein dose performed best in induction of immune response.
In summary, comprehensive qualitative assessments of the cellular immune response may allow more accurate identification of protective T-cell populations (25,26). Thus, the present study concludes that heterologous prime-boost vaccination schedules based on rBCG and Ag85B-ESAT-6 fusion protein subunit vaccine holds strong potential for future immune therapies against TB.
Acknowledgements
The present study was supported by the National 11.5 (grant nos. 2008ZX-103-013 and 2008ZX-103-011).
References
- 1.World Health Organization (WHO), corp-author Global tuberculosis control: Surveillance, planning, financing: WHO report 2008. WHO; Geneva: 2008. [Google Scholar]
- 2.Maher D, Watt CJ, Williams BG, Raviglione M, Dye C. Tuberculosis deaths in countries with high HIV prevalence: what is their use as an indicator in tuberculosis programme monitoring and epidemiological surveillance? Int J Tuberc Lung Dis. 2005;9:123–127. [PubMed] [Google Scholar]
- 3.Cohn DL, Bustreo F, Raviglione MC. International Union Against Tuberculosis and Lung Disease: Drug-resistant tuberculosis: Review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. Clin Infect Dis. 1997;24:S121–S130. doi: 10.1093/clinids/24.Supplement_1.S121. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 4.Fine PE. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SHE. Envisioning future strategies for vaccination against tuberculosis. Nat Rev Immunol. 2006;6:699–704. doi: 10.1038/nri1920. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SHE. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.McShane H, Pathan AA, Sander CR, Goonetilleke NP, Fletcher HA, Hill AV. Boosting BCG with MVA85A: The first candidate subunit vaccine for tuberculosis in clinical trials. Tuberculosis (Edinb) 2005;85:47–52. doi: 10.1016/j.tube.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Goonetilleke NPMH, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 9.Ferraz JC, Stavropoulos E, Yang M, Coade S, Espitia C, Lowrie DB, Colston MJ, Tascon RE. A heterologous DNA priming-Mycobacterium bovis BCG boosting immunization strategy using mycobacterial Hsp70, Hsp65, and Apa antigens improves protection against tuberculosis in mice. Infect Immun. 2004;72:6945–6950. doi: 10.1128/IAI.72.12.6945-6950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palendira U, Spratt JM, Britton WJ, Triccas JA. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine. 2005;23:1680–1685. doi: 10.1016/j.vaccine.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Zhu B, Wang Q, Chen J, Qie Y, Wang J, Wang H, Wang B, Wang H. Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFN-γ confers effective protection against Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol Med Microbiol. 2007;51:480–487. doi: 10.1111/j.1574-695X.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang BL, Xu Y, Wu CQ, Xu YM, Wang HH. Cloning, expression, and refolding of a secretory protein ESAT-6 of Mycobacterium tuberculosis. Protein Expr Purif. 2005;39:184–188. doi: 10.1016/j.pep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Wang B, Chen J, Wang Q, Zhu B, Shen H, Qie Y, Wang J, Wang H. Chimaeric protein improved immunogenicity compared with fusion protein of Ag85B and ESAT-6 antigens of Mycobacterium tuberculosis. Scand J Immunol. 2006;64:476–481. doi: 10.1111/j.1365-3083.2006.01812.x. [DOI] [PubMed] [Google Scholar]
- 14.Romano M, Rindi L, Korf H, Bonanni D, Adnet PY, Jurion F, Garzelli C, Huygen K. Immunogenicity and protective efficacy of tuberculosis subunit vaccines expressing PPE44 (Rv2770c) Vaccine. 2008;26:6053–6063. doi: 10.1016/j.vaccine.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Jung YJ, LaCourse R, Ryan L, North RJ. Evidence inconsistent with a negative influence of T helper 2 cells on protection afforded by a dominant T helper 1 response against Mycobacterium tuberculosis lung infection in mice. Infect Immun. 2002;70:6436–6443. doi: 10.1128/IAI.70.11.6436-6443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- 18.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14:185–191. doi: 10.1016/S1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 20.Tully G, Kortsik C, Höhn H, Zehbe I, Hitzler WE, Neukirch C, Freitag K, Kayser K, Maeurer MJ. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J Immunol. 2005;174:2174–2184. doi: 10.4049/jimmunol.174.4.2174. [DOI] [PubMed] [Google Scholar]
- 21.Brookes RH, Pathan AA, McShane H, Hensmann M, Price DA, Hill AV. CD8+ T cell-mediated suppression of intracellular Mycobacterium tuberculosis growth in activated human macrophages. Eur J Immunol. 2003;33:3293–3302. doi: 10.1002/eji.200324109. [DOI] [PubMed] [Google Scholar]
- 22.Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. ESAT-6 proteins: Protective antigens and virulence factors? Trends Microbiol. 2004;12:500–508. doi: 10.1016/j.tim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 23.D'Souza S, Rosseels V, Romano M, Tanghe A, Denis O, Jurion F, Castiglione N, Vanonckelen A, Palfliet K, Huygen K. Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun. 2003;71:483–493. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–795. doi: 10.1128/IAI.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173:5372–5380. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 26.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: Importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]