Abstract
Propolis is a bee product that has been extensively used in alternative medicine and recently has gained interest on a global scale as an essential ingredient of healthy foods and cosmetics. Propolis is also considered to improve human health and to prevent diseases such as inflammation, heart disease, diabetes and even cancer. However, the claimed effects are anticipated to be correlated to its chemical composition. Since propolis is a natural product, its composition is consequently expected to be variable depending on the local flora alignment. In this work, we present the development of a novel HPLC-PDA-ESI/MS targeted method, used to identify and quantify 59 phenolic compounds in Greek propolis hydroalcoholic extracts. Amongst them, nine phenolic compounds are herein reported for the first time in Greek propolis. Alongside GC-MS complementary analysis was employed, unveiling eight additional newly reported compounds. The antioxidant activity study of the propolis samples verified the potential of these extracts to effectively scavenge radicals, with the extract of Imathia region exhibiting comparable antioxidant activity to that of quercetin.
Introduction
Propolis is a natural product that belongs to the great family of bee products. The word propolis is a complex term originating from two ancient Greek words: pro- standing for “before or in defense” and polis meaning city. Thus, in apiculture, its meaning refers to the harboring of the hive. Propolis is a sticky, resinous substance, collected from various floral sources that are transformed and used by honeybees to construct and maintain their hives by sealing holes in their honeycombs. It is also used for smoothing out the internal walls and shelter the entrance of the hive from intruders. Trends and development in propolis research have been reviewed by Bankova [1]. In this view, the essential point in any research conducted is the chemical variability of propolis attributed to the diversity of its plant origin [2]. Propolis is a traditional remedy in alternative medicine that has been used for centuries in Egypt, Greece, and other countries as well. Propolis possesses antimicrobial [3], anti-oxidative, anti-ulcer, immunomodulatory [4] and anti-tumor activities, and the latter is proved by a plethora of reports. An informative review article on the biological activity of bee propolis in health and disease was published by Lofty in 2006 [5], collecting a significant number of research results on various medicinal aspects.
Within this context, its biological activity is attributed to its chemical composition that encompasses mainly, phenolic compounds [6]. Plant polyphenols are known for their beneficial effect on health that is vastly described for oral health (indicatively see [7]). In this regard, many research groups have presented reviews and original reports on the beneficial effect on human health of many of its constituents [8–14]. In this context, some of its components have been shown to attenuate apoptosis in rat models (for pinocembrin see [15]). Caffeic acid phenethyl ester (CAPE) is an exemplary bioactive component of propolis, exhibiting a diversity of bioactivities, such as anti-tumor effects in pre-clinical models of human breast cancer or inhibition of growth of breast cancer stem cells [16,17]. In addition, many propolis components exist in some specific plant extracts as well and have been studied for their anti-cancer activity (see indicatively [18]). The chemical base of the biological activity of flavonoids, exemplified by their antioxidant properties, are collectively presented by Kancheva and Kasaikina [19].
Until recently, only three works have dealt exclusively with the phenolic composition of propolis extracts from Greece and Cyprus. Kalogeropoulos et al. used GC-MS analysis after derivatization reaction [3] to explore its chemical profile. An additional work was published by Graikou et al., using GC-MS again, to highlight the features of Meditteranean propolis, including samples from Greece, Cyprus, Croatia and Algeria [20]. The other was reported by Lagouri et al. in 2014, including a limited amount of compounds [21]. On international scale an indicative landmark targeted study was reported by Falcao et al., incorporating almost 40 analytes, that managed to efficiently display the chemical profile of Portuguese propolis [22].
Considering the importance of propolis due to its pharmacological properties, and the limited number of works on Greek propolis, we decided to revisit its chemical composition and assess the antioxidant activity of the studied extracts. The latter was reinforced by the demographics of the European agricultural industry that render Greece second regarding bee colonies number [23], designating a substantial potential for the exploitation of this matrix.
Therefore and considering that, to our knowledge, none targeted high-performance liquid chromatographic mass spectrometric (HPLC-MS) method analysis work on phenolic compounds of Greek propolis is reported, a multi-analyte HPLC-MS (using electronspray interface and diode array, HPLC-ESI-PDA/MS) method to monitor and quantify 59 compounds belonging to relevant bioactive chemical categories, such as aromatic acids and flavonoids, was developed. The selection of compounds included in the HPLC-PDA-ESI/MS method was based on previously published works on propolis, aiming to incorporate as many as possible compounds some of which were previously not described in Greek propolis. Furthermore, Artepillin C that is a unique constituent of Brazilian propolis was incorporated, although not expected to be detected. The method was applied to the analysis of eight propolis samples from Greece and to a Brazilian tincture propolis. Tentative characterization of new compounds via HPLC-PDA-ESI/MS under full scan mode was also pursued and reported. In addition, the use of GC-MS was implemented on a complementary basis, despite its extensive use in previous works. Last but not least, the propolis extracts were assessed for their antioxidant activity using a standard protocol. Such extracts, in previous works, have been evaluated extensively for their antioxidant activity exhibiting high radical scavenging activity (indicatively see [24]). Overall, both chromatographic methods revealed several new constituents, while one of the propolis extracts displayed comparative antioxidant activity to that of the bioactive molecule of quercetin. Finally, statistical analysis demonstrated certain correlations among the variables selected.
Materials and Methods
Chemicals and Reagents
The specific compounds, were: Caffeic acid, CAPE, chrysin, luteolin, daidzein, suberic acid, apigenin, (Alfa Aesar), pinocembrin, isorhamnetin, isosakuranetin, vitexin, orientin, rosmarinic acid, myricetin, vanillin, ursolic acid, hydroxytyrosol, tangeretin, chrysoeriol, betulinic acid, eriodictyol, sakuranetin, naringenin, t-cinnamic acid, genistein, diosmetin, resveratrol, galangin, pinocembrin 7-methyl ether, techtochrysin, (Extrasynthese) rutin, isoferulic acid, ellagic acid, kaempferol, quercetin, corosolic acid, acacetin, diosmin, protocatechuic acid ethyl ester, hesperetin, phloridzin, chlorogenic acid, p-coumaric acid, (±)catechin, maslinic acid, (Sigma Aldrich), rhamnetin, syringic acid, protocatechuic acid, ferulic acid, kaempferide, adipic acid, pinostrobin, gallic acid, pinobanksin (Fluka), naringin, hesperidin, (Acros Organics) pinobanksin-3O-acetate (Interchim Inc), cinnamylidenacetic acid, artepillin C (Wako Chemicals). o-Orselllinaldehyde was purchased from Santa Cruz Biotechnology (USA).
Water, acetonitrile, methanol and formic acid were purchased from Fisher Scientific, UK and they were of LC-MS grade. Ethanol was purchased from Merck, Germany. PTFE filters (0.45 μm) were obtained from Macherey-Nagel, Germany.
Samples
The propolis samples were obtained directly from eight individual beekeepers from several regions/locations of Greece. Nafplio (Argolida, Eastern Peloponnese), Amorgos (Cyclades Islands, Aegean Sea), Crete (Heraklion, Central Crete), Kos (Dodecanese Islands, South Aegean), Lakonia (Areopoli, Southern Peloponnese), Imathia (Central Macedonia, North Greece), Arkadia (Central Peloponnese) and Corfu island (Ionian Sea). The samples were obtained after the honey-harvesting season, by scraping as indicated by the local beekeepers. The propolis samples were collected during 2014–2015, and they were stored at -20°C before extraction. Finally, a Brazilian green propolis tincture (commercially available) was obtained and analyzed to verify method’s ability to detect and quantify, Artepillin C, its most bioactive component, which is unique for Brazilian propolis.
Preparation of Extracts
Raw, crude propolis was cut into very small pieces and was homogenized. Then, 10 mL of a 4:1 mixture solution of ethanol-water respectively, was added to 1 g of the propolis, and the mixture was continuously stirred for 24 h at room temperature, in dark. Subsequently, the crude mixture was transferred to a falcon tube and subjected to centrifugation (Heraeus Labofuge 400R Thermo Electron Corporation, 5 min, 10°C, and 4000 rpm). The upper liquid layer was then decanted, filtered, and stored at -20°C overnight. The latter favored the removal of waxes (due to their precipitation). The above procedure was repeated to ensure quantitative extraction of compounds. The resulting combined solution was filtered, evaporated to near dryness and then freeze-dried. The derived dry extract was reconstituted in a 4:1 ethanol-water solution. Before injection into the HPLC-ESI-PDA/MS system, the extract was diluted with methanol to avoid contamination of the mass spectrometer and concentrations to fall within the calibration curve range, and finally filtrated using PTFE filter. For GC-MS analysis pure EtOH was used as extraction medium, and the duration of extraction was lowered to 5 h. EtOAc extraction was also applied for 5 h, with not substantial differences in terms of compounds extracted compared to EtOH.
High-Performance Liquid Chromatography-Electrospray Photo Diode Array Mass Spectrometry
A Shimadzu (Kyoto, Japan) LCMS-2010 EV Liquid Chromatograph Mass Spectrometer instrument was used with the LCMS solution version 3.0 software consisting of an SIL-20A prominence autosampler and an SPD-M20A diode array detector. The latter were coupled in series with a mass selective detector equipped with an atmospheric pressure ionization. The LC separation was achieved on a Zorbax Eclipse Plus, 3.5 μm, 150 × 2.6 mm i.d. chromatographic column. The mobile phase consisted of two channels, channel (A) 0.1% formic acid in water (A) and channel (B) pure acetonitrile (B). The flow rate was set at 0.3 mL•min-1 and the column gradient program started at 20% B, and ramped linearly over the course of 10 min at 30% B. Subsequently the system was linearly increased until the 40 min to 40% B, where it was maintained up to the 70th min. Then, the acetonitrile percentage was linearly increased over 25 min to 70%, and kept for additional 5 min. Then, acetonitrile returned in the course of 5 min at the initial concentration of 20%, where it stayed for additional 3 min. Overall runtime was extended to 108 min, albeit the majority of compounds elute before the 50th minute. Electron Spray Ionization (ESI) mode (using discrete events for each analyte monitored) was utilized, functioning in the selected ion monitoring mode (SIM). Photodiode array monitored wavelengths from 190 to 800 nm.
Validation of the Present Method—Quantification of Constituents
The developed method was validated following mainly the International Conference on Harmonization [25] also considering the SANCO document [26]. A review publication, pertinent to chemical measurements in natural products research was also regarded [27]. Validation study was performed regarding recovery, linearity, intra-day and inter-day precision. The calibration curves were established using the dilute standard solution of the 59 compounds of the method. Calibration curves ranged from 40 to 5000 ng/mL. The selected range was decided considering three parameters: a) the coverage of expected concentrations usually encountered for these compounds after extraction, sample preparation and dilution, b) the MS detector protection from high concentration levels and c) the analytical performance of the method. Blank experiments were also conducted (complete procedure without matrix extract). Standard addition was used for the recovery study at two concentration levels.
In this context, the precision of the chromatographic method was expressed as the RSD % of the repeatability (intra-day) and intermediate precision (inter-day) analyses (n = 3) over 1, 2 and 3 days. Repeatability and intermediate precision were considered acceptable when relative standard deviation values (RSD%) were < 20%. LOQs were defined as the lowest validated spiked level that met the method performance acceptability criteria, regarding mean recoveries in the range of 70–120%, with RSDr 20%.
To estimate if the matrix impacts considerably the peak area and, therefore, the sensitivity of the analytes, the slopes of the calibration lines obtained for propolis after standard addition (bmatrix) and the solvent (bsolvent) were divided to determine the matrix factor and the % matrix effect (ME) was calculated by Eq (1).
| (1) |
GC-MS Conditions
The GC-MS analysis was performed on a Chromtech Evolution MS/MS triple quadrupole mass spectrometer built on an Agilent 5975 B inert XL EI/CI MSD system that was operated in full scan data acquisition mode. Samples were injected with a Gerstel MPS-2 autosampler using a 10-μL syringe. Separations were performed on an HP-5ms UI, length 30m, ID 0.25mm, film thick. 0.25 μm (J&W Folsom, USA). Helium was used as the carrier gas at a flow rate of 1.2 mL min-1. The column oven temperature program started from 80°C, staying for 3 min, increased to 160°C at a rate of 8°C min-1 where it remained for 10 min, then increased to 220°C at a rate of 13°C min-1 and held for 5 min. Then the temperature was raised to 260°C min-1 at a rate of 5°C min-1, held for 10 min and finally ramped to 300°C at a rate of 5°C min-1 held for 2 min. The transfer line, manifold, and source of ionization temperatures were 300, 40 and 230°C. The electron multiplier voltage was set at 2000 V. The total GC analysis lasted for 56.62 min.
Identified peaks in GC-MS were confirmed by comparing the acquired mass spectra with those in the commercial library of NIST 08.
DPPH Radical Scavenging Assay
The selected concentrations of the ethanolic extracts were assessed in the range of 0.05 to 250 μg/mL. The latter was established after initial testing of indicative levels so as to determine the final work range depending on the % antioxidant activity. Sample stock solutions of 1 mg/mL were diluted to the final concentration of ethanol. A 0.3 mM DPPH ethanol solution was prepared in absolute ethanol. The experimental part consisted of mixing of 1 mL of DPPH solution with 2.5 mL of the propolis extract [28, 29]. Then, after a gentle stirring of 1 min, the solution was left for 30 min at room temperature so as the extract to react with DPPH and scavenge it. The absorbance was measured at 520 nm, in a spectrophotometer (Shimadzu, UV-VIS, Pharma-Spec 1700). Ethanol was utilized as a negative control while quercetin was used as positive control. Control was composed of ethanol (2.5 mL) and DPPH radical solution (1 mL). All the analyses were carried out in triplicate.
Total Phenolic Content
Folin Ciocalteau is a mixture of phosphomolybdate and phosphotungstate utilized for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants [30,31]. In this context, it was used for the determination of total phenolic content (TPC). Briefly, 20 μL of the propolis extract (1 mg/mL) were sequentially mixed with 300 μL of distilled water and 100 μL of Folin Ciocalteau’s phenol reagent. After 4 min, 1000 μL of distilled water and 400 μL of 20% sodium carbonate were added. The reaction mixture was kept in the dark for 2 h at room temperature and absorbance was measured at 760 nm in a spectrophotometer (Shimadzu, UV-VIS, Pharma-Spec 1700). TPC was calculated from the calibration curve generated from standard solutions of gallic acid ranging from 0.5 to 20 μg/mL (y = 0.0082x, r2 = 0.9980, intercept = 0.0018). The latter was expressed as gallic acid equivalent (mg) per gram of extract (mg GAE/g dry extract). Blank was prepared as above using ethanol instead of propolis extract. All analyses were performed using three aliquots of each extract sample, measured in triplicate, calculating the average value.
Total Flavonoids Content
Total flavonoid contents (TFC) were determined by the aluminium colorimetric method [32], using quercetin as reference standard [33]. More specifically, in an aliquot (100 μL) of propolis extract (1 mg mL-1) was sequentially added 1 mL of methanol, 3.5 mL of HPLC water, 2% (w/v) AlCl3 (200 μL) and 1 M potassium acetate (200 μL). After 30 min of incubation at room temperature, the absorbance was measured at 435 nm by a spectrophotometer. The TFC was expressed as μg of quercetin equivalent per mg of dry weight of the extract. All analyses were performed using three aliquots of each extract sample, measured in triplicate, calculating the average value.
Principal Component Analysis
The analysis was performed with SPSS 20.0 (IBM Corp.). All data were tested as for whether they were normally distributed by the Shapiro-Wilk’s test and the visual inspection of data’s histograms, normal Q-Q plots and box plots. Further, data were tested for equality of variances (homoscedasticity) by the Levene’s test. For those samples, which failed to comply with the conditions of normality and homoscedasticity, the logarithmic transformation log(x+1) was applied.
For the DPPH derived IC50 values, lower values reflect higher antioxidant activity. Therefore, for the PCA purpose, all values were normalized by setting the lowest IC50 value as 100, and rest of values were adjusted accordingly.
Results
Chromatographic Separation
The developed HPLC-PDA-ESI/MS method managed to separate with substantial resolution the majority of targeted analytes (see Fig 1). Considering that quantitative analysis was performed predominantly using the SIM mode that monitors few mass to charge values (m/z) depicted in Table 1, and the background is reduced, the efficient separation of all analytes amongst them was not a prerequisite. Nevertheless, to provide better resolution for compounds eluting from 1.5 min to 2.1 min a modification of the mobile phase was implemented. Hence, an increase of water in the mobile phase resulted in delayed elution and enhanced resolution especially for the compounds eluting between 1.5–2 min (see Fig 2).
Fig 1. Magnified HPLC-UV chromatogram of a standard solution (500 ng/mL), at 254 nm.
Table 1. Characterization of phenolic compounds by HPLC-DAD-ESI/MS.
| Compound Number | Compound Name | tR (min) | λmax (nm) | Quantitation ion | Confirmation ion(s) | Mode polarity | Voltage (kV) | Event N° |
|---|---|---|---|---|---|---|---|---|
| 1 | Protocatechuic acid | 2.1 | 260, 294 | 153 | 109 | ESI(-) | 2.1 | Event 1 |
| 2 | Pinocembrin | 30.4 | 289 | 255 | 213 | ESI(-) | 1.6 | Event 2 |
| 3 | Kaempferol | 16.3 | 264, 366 | 285 | 151 | ESI(-) | 2.1 | Event 3 |
| 4 | Apigenin | 15.3 | 268, 337 | 269 | 225, 151 | ESI(-) | 1.8 | Event 4 |
| 5 | Chrysin | 28.2 | 268 | 253 | 209 | ESI(-) | 1.6 | Event 5 |
| 6 | Galangin | 31.4 | 265, 356 | 269 | 241, 227 | ESI(-) | 1.6 | Event 6 |
| 7 | Chlorogenic acid | 2.1 | 325 | 353 | 191 | ESI(-) | 2.0 | Event 7 |
| 8 | Daidzein | 9.5 | 255 | 253 | 208 | ESI(-) | 2.1 | Event 8 |
| 9 | Ellagic acid | 3.6 | 252, 367 | 301 | 145 | ESI(-) | 1.8 | Event 9 |
| 10 | Ferulic acid | 4.6 | 295 | 193 | 134 | ESI(-) | 2.0 | Event 10 |
| 11 | Gallic acid | 1.8 | 280 | 169 | 125 | ESI(-) | 2.1 | Event 11 |
| 12 | Hesperetin | 16.4 | 340 | 300.8 | 163.9 | ESI(-) | 1.9 | Event 12 |
| 13 | Hydroxytyrosol | 2.0 | 277 | 153 | - | ESI(-) | 2.3 | Event 13 |
| 14 | Luteolin | 11.7 | 268, 349 | 285 | 241 | ESI(-) | 2.1 | Event 14 |
| 15 | p-Coumaric acid | 3.9 | 309 | 163 | 119 | ESI(-) | 2.4 | Event 15 |
| 16 | Pinobanksin | 15.4 | 291, 330 | 271 | 253, 225 | ESI(-) | 2.4 | Event 16 |
| 17 | PIN-7ME | 57.7 | 286 | 271 | 270 | ESI(+) | 1.6 | Event 17 |
| 18 | Quercetin | 11.6 | 255, 370 | 301 | 151 | ESI(-) | 1.8 | Event 18 |
| 19 | Tectochrysin | 53.2 | 268 | 269 | 226 | ESI(+) | 2.2 | Event 19 |
| 20 | Caffeic acid | 2.5 | 291, 321 | 179 | 135 | ESI(-) | 1.6 | Event 20 |
| 21 | Sakuranetin | 29.6 | 284 | 285 | 286 | ESI(-) | 2.4 | Event 21 |
| 22 | Rhamnetin | 22.1 | 255, 367 | 315 | 300, 193 | ESI(-) | 1.8 | Event 22 |
| 23 | CAPE | 33.5 | 245, 298, 325 | 283 | 179, 135 | ESI(-) | 1.8 | Event 23 |
| 24 | Pinostrobin | 66.9 | 289 | 271 | 167, 131 | ESI(+) | 2.3 | Event 24 |
| 25 | Syringic acid | 2.0 | 273, 217 | 197 | 182 | ESI(-) | 2.6 | Event 25 |
| 26 | Kaempferide | 32.5 | 335, 265 | 299 | 284, 151 | ESI(-) | 2.0 | Event 26 |
| 27 | Acacetin | 27.8 | 268 | 283 | 269 | ESI(-) | 2.0 | Event 27 |
| 28 | Rutin | 2.7 | 255, 354 | 609 | 301, 273 | ESI(-) | 2.0 | Event 28 |
| 29 | Protocatechuic acid ethyl ester | 7.3 | 260, 222, 294 | 181 | 182, 153 | ESI(-) | 2.0 | Event 29 |
| 30 | Resveratrol | 8.5 | 305, 216 | 227 | 184.9 | ESI(-) | 2.0 | Event 30 |
| 31 | Phloridzin | 7.5 | 282, 234 | 435 | 273 | ESI(-) | 2.0 | Event 31 |
| 32 | Maslinic acid | 76.6 | 277 | 471 | 453, 425 | ESI(-) | 2.1 | Event 32 |
| 33 | Naringenin | 14.6 | 288, 233 | 271 | 119 | ESI(-) | 1.9 | Event 33 |
| 34 | Eriodictyol | 10.7 | 287, 233 | 287 | 151 | ESI(-) | 2.1 | Event 34 |
| 35 | Diosmetin | 15.7 | 347, 246 | 299 | 284, 256 | ESI(-) | 2.0 | Event 35 |
| 36 | Rosmarinic acid | 6.6 | 327, 236 | 359 | 161 | ESI(-) | 2.5 | Event 36 |
| 37 | Myricetin | 6.8 | 372, 253 | 317 | 178.9 | ESI(-) | 1.9 | Event 37 |
| 38 | Isorhamnetin | 16.5 | 371, 254 | 315 | 301 | ESI(-) | 2.0 | Event 38 |
| 39 | Isosakuranetin | 29.2 | 288, 234 | 285 | 178.9 | ESI(-) | 2.0 | Event 39 |
| 40 | (+)-Catechin | 1.6 | 280 | 291 | - | ESI(+) | 2.5 | Event 40 |
| 41 | Orientin | 2.5 | 348, 254 | 447 | - | ESI(-) | 2.0 | Event 41 |
| 42 | Vitexin | 2.8 | 360, 268 | 431 | 283 | ESI(-) | 1.5 | Event 42 |
| 43 | trans-Cinnamic acid | 12.1 | 276 | 149 | - | ESI(+) | 2.0 | Event 43 |
| 44 | Pinobanksin 3-O-acetate | 33.0 | 280 | 313 | 253 | ESI(-) | 2.2 | Event 44 |
| 45 | Cinnamylideneacetic acid | 71.2 | 229, 276 | 219 | - | ESI(-) | 2.4 | Event 45 |
| 46 | Artepillin C | 63.2 | 315 | 299 | 255 | ESI(-) | 2.1 | Event 46 |
| 47 | Adipic acid | 2.9 | 210 | 145 | 126.8, 100.9 | ESI(-) | 2.3 | Event 47 |
| 48 | Ursolic acid | 100.4 | 215 | 439 | 411, 457 | ESI(+) | 2.3 | Event 48 |
| 49 | Suberic acid | 3.2 | - | 173.1 | - | ESI(-) | 2.5 | Event 49 |
| 50 | Genistein | 14.2 | 260 | 269 | 133 | ESI(-) | 2.1 | Event 50 |
| 51 | Hesperidin | 5.1 | 281, 229 | 609 | - | ESI(-) | 2.2 | Event 51 |
| 52 | Corosolic acid | 77.0 | - | 471.4 | 425 | ESI(-) | 2.2 | Event 52 |
| 53 | Betulinic acid | 97.3 | - | 455.5 | - | ESI(-) | 2.0 | Event 53 |
| 54 | Isoferulic acid | 4.8 | 323, 240 | 195 | 177 | ESI(+) | 2.4 | Event 54 |
| 55 | Naringin | 4.9 | 283 | 579 | - | ESI(-) | 1.4 | Event 55 |
| 56 | Tangeretin | 34.4 | 325, 270 | 373.1 | 343.3 | ESI(+) | 1.8 | Event 56 |
| 57 | Diosmin | 5.3 | 345, 253 | 607 | - | ESI(+) | 1.8 | Event 57 |
| 58 | Vanillin | 4.1 | 230, 279 | 153 | - | ESI(+) | 2.5 | Event 58 |
| 59 | Chrysoeriol | 15.1 | 348, 254 | 298.9 | 283.9 | ESI(-) | 2.2 | Event 59 |
Fig 2. HPLC-UV chromatogram (at 254 nm) of standard solution mix (500 ng/mL) with delayed elution of compounds (indicative marking).
Identification-Confirmation of Compounds
Identification and confirmation of compounds was achieved by comparing the m/z values, the retention time and UV absorption spectrum with those of the analytical standards, also considering characteristic works of the relative literature [22,34,35]. ESI ionization proved substantial in producing characteristic ions for the compounds studied (see also “Figures E and F in S1 File” and “Figures A-Z, Figures A1-Z1, and Figures A2-C2 in S2 File”). Peak purity index in the UV-spectra, as it was produced by the respective LC software (see Table 1 for maximum wavelengths obtained for each compound) assisted selectivity evaluation, regarding as pure, the peaks with spectra similarity exceeding 95%. It is noteworthy, however, that in natural products hundreds of compounds not listed in targeted analysis, can potentially co-elute with listed compounds. Therefore, caution is required in quantitative analysis. This “risk” is minimized when MS is used in line with UV, as in the presented work.
Method Validation Results
With regard to method validation characteristics, these are presented in Table 2. Linearity was checked for all analytes in the ranges of 40–5000, and 60–5000 ng/mL with acceptable correlation coefficient values (r2 ≥ 0.99). Recovery was assessed at two levels by standard addition of the standards mix solutions to the samples (in triplicate), and it was above 70%. LOQs were determined at 40 and 60 ng/gextract for two sets of compounds as presented in Table 2, fulfilling the respective criteria (as mentioned above). Concerning matrix effect, slight signal suppression was evidenced for the majority of analytes (see Table 3 for ME results). However, the %ME, never surpassed the threshold value of ±20%, which is considered low based on proposed literature classification [36].
Table 2. Analytical Method Validation Characteristics.
| Calibration Range 40–5000 (ng/mL) | |||||||
| Analyte | Regression Equation | Regression coefficient (R2) | ME (%) | Recovery ±RSD % | Inter-d precision | Intra-d-precision | |
| n = 3 | RSD % n = 3 | ||||||
| 100 ng/g | 1000 ng/g | 100 ng/g | 100 ng/g | ||||
| Apigenin | y = 17118x+735090 | 0.9962 | -2.2 | 82±10 | 77±15 | 3.40 | 3.02 |
| Chrysin | y = 4074,4x-14955 | 0.9991 | -1.9 | 94±10 | 93±14 | 1.05 | 1.65 |
| Galangin | y = 3603,9x-5504,1 | 0.9993 | -0.8 | 90±15 | 87±9 | 3.30 | 2.56 |
| Ellagic acid | y = 4181,8x+459836 | 0.9900 | -3.8 | 85±11 | 87±13 | 2.18 | 4.02 |
| Ferulic acid | y = 5287x+461871 | 0.9947 | -5.2 | 81±14 | 73±9 | 3.05 | 5.04 |
| Hesperetin | y = 7799,5x+407655 | 0.9945 | -1.1 | 83±10 | 90±12 | 1.09 | 1.95 |
| Luteolin | y = 103735x-4000000 | 0.9926 | -0.9 | 78±11 | 80±8 | 3.78 | 3.14 |
| p-Coumaric acid | y = 6176,9x+209484 | 0.9985 | -4.4 | 73±5 | 79±6 | 2.89 | 2.49 |
| Pinobanksin | y = 8394,7x+299561 | 0.9970 | -6.9 | 79±10 | 76±7 | 1.67 | 1.77 |
| Quercetin | y = 5472,7x-28037 | 0.9993 | -7.2 | 93±12 | 85±9 | 5.39 | 4.72 |
| Caffeic acid | y = 1729,9x-63292 | 0.9968 | -0.6 | 69±10 | 69±7 | 2.84 | 2.94 |
| CAPE | y = 24466x+782287 | 0.9951 | -8.1 | 91±13 | 88±10 | 4.04 | 4.89 |
| Rhamnetin | y = 13067x-578824 | 0.9959 | -2.3 | 80±14 | 74±5 | 5.40 | 5.15 |
| Kaempferol | y = 60404x-593055 | 0.9976 | -1.8 | 78±6 | 84±7 | 0.80 | 2.42 |
| Chlorogenic acid | y = 26764x-709939 | 0.9995 | -7.7 | 76±7 | 80±10 | 3.02 | 5.25 |
| Protocatechuic acid | y = 437,1x+21073 | 0.9992 | -0.9 | 80±14 | 88±16 | 2.47 | 2.85 |
| Syringic acid | y = 3901,6x-18827 | 0.9991 | -2.3 | 80±12 | 77±6 | 3.17 | 6.01 |
| Daidzein | y = 63929x+3000000 | 0.9929 | 1.2 | 80±12 | 86±5 | 1.44 | 1.65 |
| Kaempferide | y = 23465x-123085 | 0.9919 | 0.4 | 79±13 | 82±12 | 1.28 | 2.34 |
| Acacetin | y = 9665,1x+603921 | 0.9933 | -8.3 | 88±7 | 79±9 | 3.54 | 2.91 |
| Resveratrol | y = 102223x-632009 | 0.9988 | -2.9 | 81±7 | 79±10 | 7.01 | 6.72 |
| Naringenin | y = 6670,4x+39019 | 0.9928 | -8.1 | 85±12 | 91±14 | 8.11 | 4.38 |
| Adipic acid | y = 8841,6x-42008 | 0.9972 | -7.5 | 89±10 | 82±14 | 6.21 | 3.26 |
| Betulinic acid | y = 44586X-311102 | 0.9956 | -4.0 | 89±15 | 79±5 | 1.98 | 3.29 |
| Cinnamylidene acetic acid | y = 22654x-188755 | 0.9991 | -2.9 | 95±10 | 89±5 | 1.17 | 1.29 |
| Pinobanksin 3O-acetate | y = 1333,7x-7987 | 0.9972 | -6.9 | 77±3 | 83±4 | 3.23 | 3.04 |
| Vitexin | y = 40983x-283008 | 0.9987 | 0.8 | 95±7 | 83±4 | 1.01 | 3.24 |
| Orientin | y = 41202x+326081 | 0.9919 | 0.9 | 85±7 | 81±10 | 4.45 | 3.74 |
| Isosakuranetin | y = 5565,7x-200093 | 0.9966 | -3.6 | 91±14 | 81±6 | 4.05 | 2.91 |
| Myricetin | y = 11123x-509882 | 0.9933 | -1.3 | 91±7 | 84±9 | 6.01 | 2.31 |
| Rosrmarinic acid | y = 203347x-550899 | 0.9944 | -1.4 | 83±7 | 84±12 | 4.11 | 2.78 |
| Genistein | y = 1009,8x-16789 | 0.9992 | -8.4 | 78±5 | 92±13 | 5.21 | 2.44 |
| Tangeretin | y = 37768,4x-309887 | 0.9982 | -1.1 | 83±7 | 88±9 | 1.12 | 3.01 |
| Diosmin | y = 15006χ+201910 | 0.9963 | -2.7 | 92±11 | 86±8 | 2.23 | 3.71 |
| Diosmetin | y = 4571,1χ-16730 | 0.9909 | -1.9 | 92±7 | 79±9 | 1.08 | 1.99 |
| Isorhamnetin | y = 55601x+320985 | 0.9948 | 0.7 | 80±5 | 80±8 | 1.29 | 3.61 |
| Hydroxytyrosol | y = 4201,8x-89002 | 0.9917 | -4.9 | 91±9 | 80±11 | 4.04 | 2.17 |
| Vanillin | y = 16723x-236489 | 0.9917 | 1.1 | 83±13 | 90±14 | 1.10 | 1.38 |
| Calibration Range 60–5000 (ng/mL) | |||||||
| Recovery ±RSD % | Inter-d precision | Intra-d-precision | |||||
| n = 3 | RSD % n = 3 | ||||||
| 100 ng/g | 1000 ng/g | 100 ng/g | 100 ng/g | ||||
| Gallic acid | y = 1496,8x-63708 | 0.9972 | 1.3 | 84±7 | 83±10 | 2.94 | 3.47 |
| Pinocembrin | y = 3049,9x-44337 | 0.9960 | -0.8 | 83±11 | 87±9 | 2.20 | 2.85 |
| PIN-7ME | y = 329,7x+21307 | 0.9997 | -2.2 | 84±10 | 79±9 | 5.39 | 4.97 |
| Pinostrobin | y = 33166x+4000000 | 0.9976 | -6.8 | 91±7 | 85±12 | 1.55 | 1.82 |
| Tectochrysin | y = 1300,6x-16230,5 | 0.9999 | -3.5 | 81±10 | 77±6 | 5.26 | 4.82 |
| Sakuranetin | y = 1240,2x-27612 | 0.9986 | -3.2 | 91±9 | 88±8 | 1.06 | 3.76 |
| Rutin | y = 875,5x+11099 | 0.9984 | -0.8 | 80±3 | 85±9 | 2.78 | 2.98 |
| Maslinic acid | y = 3210,1x-88702 | 0.9947 | 0.5 | 77±8 | 87±12 | 5.02 | 6.03 |
| Phloridzin | y = 7745,1x+30222 | 0.9990 | -2.7 | 91±3 | 82±8 | 2.11 | 1.98 |
| Artepillin C | y = 15557,1x-409876 | 0.9972 | -4.3 | 75±3 | 88±11 | 4.98 | 6.04 |
| Ursolic acid | y = 1349,8χ-20778 | 0.9991 | -8.2 | 79±7 | 82±14 | 3.71 | 5.11 |
| Suberic acid | y = 45520x+239044 | 0.9909 | 1.2 | 92±15 | 87±8 | 1.92 | 3.02 |
| Hesperidin | y = 6609,5x-101009 | 0.9918 | -3.9 | 90±11 | 83±6 | 3.47 | 2.19 |
| Isoferulic acid | y = 980,5x-17750 | 0.9992 | 0.6 | 91±9 | 82±12 | 3.04 | 5.19 |
| Corosolic acid | y = 30004x+220887 | 0.9990 | 0.4 | 81±10 | 79±5 | 1.55 | 4.92 |
| Eriodictyol | y = 60430x-390334 | 0.9973 | -2.5 | 101±11 | 89±7 | 3.01 | 2.84 |
| Chrysoeriol | y = 3090,5x+23098 | 0.9992 | -3.0 | 85±4 | 91±8 | 4.12 | 6.71 |
| Naringin | y = 52234x+144502 | 0.9994 | 1.0 | 79±11 | 78±8 | 4.12 | 6.71 |
Table 3. Quantitation of constituents in Greek propolis extracts.
| Content μg/g (dry extract), n = 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Analyte | Arkadia | Kerkira | Nafplio | Amorgos | Crete | Kos | Lakonia | Imathia |
| Pinocembrin | 1710.5 | 581.9 | 4381 | 361.2 | 3560 | 3944 | 1170.5 | 13992 |
| Apigenin | 120.3 | nd | 40.5 | nd | 78.8 | 939.7 | 240.8 | 1989.8 |
| Chrysin | 3490.6 | 1011.4 | 3790.2 | 246.1 | 169.7 | 1609 | 825.2 | 9940.3 |
| Galangin | 22.5 | nd | 1501 | 22.4 | 154.2 | 2589 | 756.3 | 2529.1 |
| Ellagic acid | nd | 39.8 | nd | nd | nd | nd | nd | 49.7 |
| Tectochrysin | 49.5 | nd | nd | nd | nd | nd | nd | 55.4 |
| Syringic acid | 9.3 | nd | nd | nd | 7.5 | nd | nd | nd |
| Ferullic acid | 61.8 | 73.9 | nd | nd | nd | 17.4 | 2.8 | nd |
| Gallic acid | 47.6 | nd | 263.2 | 44.9 | 33.2 | 177.1 | 61.0 | 27.9 |
| Hesperetin | nd | nd | nd | nd | 19.9 | 55.8 | nd | nd |
| Luteolin | 23.4 | nd | 336 | 25.6 | nd | 41.1 | 248.1 | 206.3 |
| p-Coumaric acid | 186.8 | nd | 846 | 36.4 | 52.5 | 1147 | 50.1 | 186.8 |
| Pinobanksin | 82.1 | nd | 130.1 | nd | 229.7 | 1235 | 326.9 | 190.0 |
| PIN-7ME | 270.9 | nd | 1018 | 280.3 | nd | nd | nd | 260.6 |
| Caffeic acid | 34.4 | nd | nd | 138.1 | 135.3 | 1673 | 228.3 | nd |
| Pinostrobin | 28.1 | nd | nd | nd | nd | nd | 679 | nd |
| CAPE | 37.7 | nd | 39.4 | 402.4 | 133.7 | 310.9 | 85.8 | nd |
| Quercetin | 43.2 | nd | 596.5 | 318.6 | nd | nd | 201.1 | nd |
| Rhamnetin | 39.3 | nd | 46.1 | nd | nd | nd | 89.1 | nd |
| Kaempferol | nd | 92.5 | 38.0 | nd | 260.7 | 179.6 | 1343 | 89.3 |
| Chlorogenic acid | nd | nd | 301.6 | 23.8 | 124.6 | 28.5 | nd | 61.1 |
| Protocatechuic acid | nd | nd | nd | 29.2 | 102.4 | 99.5 | 36.1 | 97 |
| Kaempferide | nd | nd | 3.2 | nd | nd | nd | nd | nd |
| Acacetin | nd | nd | nd | 96.3 | 226.4 | nd | 354.3 | 557.5 |
| Resveratrol | nd | nd | 0.9 | nd | nd | nd | nd | 1.4 |
| Eriodictyol | 34.1 | nd | nd | nd | nd | 932.7 | nd | 992.6 |
| Naringenin | 392.5 | nd | 721.7 | 302.9 | nd | nd | 1027 | nd |
| Pinobanksin-3o-acetate | 742 | nd | nd | 2949 | nd | nd | 1870.3 | 2809 |
| (+)-Catechin | 1641 | nd | nd | nd | nd | 297.7 | 1925 | 2717 |
| Rutin | 346.4 | 63.7 | nd | nd | nd | 237.5 | nd | nd |
| Isorhamnetin | nd | nd | 931 | nd | nd | nd | 257.3 | 151.4 |
| Sakuranetin | nd | nd | nd | nd | nd | nd | nd | 727 |
| Isosakuranetin | nd | nd | nd | nd | 76.7 | nd | 134 | nd |
| Daidzein | nd | nd | nd | nd | nd | 26.3 | nd | nd |
| Vitexin | nd | nd | nd | nd | nd | nd | nd | 30.3 |
| Rosmarinic acid | nd | 58.3 | nd | nd | nd | nd | nd | nd |
| Myricetin | nd | nd | nd | nd | 119 | nd | 479.2 | nd |
| Ursolic acid | nd | nd | nd | nd | nd | nd | 369.1 | nd |
| Genistein | 182.8 | nd | nd | nd | nd | 420.3 | nd | nd |
| Cinnamilidene acetic acid | nd | nd | nd | nd | nd | nd | 41.2 | nd |
| t-Cinnamic acid | 1.8 | 132.6 | nd | nd | 71.7 | nd | nd | 217.7 |
| Vanillin | nd | 101.7 | nd | 83.9 | nd | nd | nd | nd |
± standard deviation (SD) was less than 10%, nd, non detected
Quantitation of Constituents
The analysis of eight Greek propolis samples from Crete, Kos, Kerkira and Amorgos islands, Arkadia, Lakonia, Imathia, and Nafplio regions revealed the presence of several phenolic compounds (indicatively for Crete see Figs 3, 4 and 5, for Imathia Fig 6, and representative chromatograms in supplementary material “Figures C-D in S1 File”). Amongst them, various new constituents, namely isosakuranetin, luteolin, rhamnetin, hesperetin, acacetin, kaempferide, eriodictyol, and pinostrobin were detected. All the above components are reported for the first time in analyzed Greek propolis samples. Quantitative results for each region are depicted in Table 3, while in Figs 7 and 8 the distribution of components is portrayed (both as chemical classes and as individual compounds). Quantitation was performed with the HPLC-ESI/MS methodology; results, however, were verified with UV as well (particularly for the most abundant compounds). In this context, the six most dominant compounds for all regions were pinocembrin, chrysin, galangin, apigenin, pinobanksin 3-O-acetate, and (±)catechin that is in accordance with literature for European propolis major polyphenolic constituents (indicatively see [3,22, 34,37]).
Fig 3. HPLC-UV chromatogram of Crete propolis extract at 280 nm.
Fig 4. SIM chromatogram showing quantitation ions for kaempferol and pinocembrin (Crete sample).
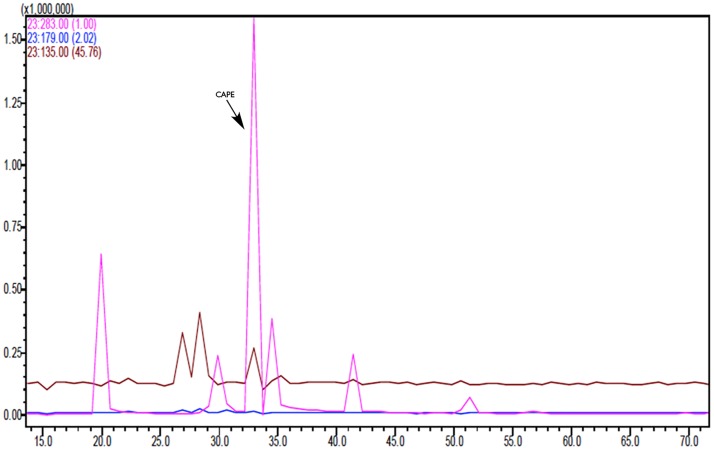
Fig 5. SIM chromatogram showing quantitation and confirmation ions for CAPE (Crete sample).
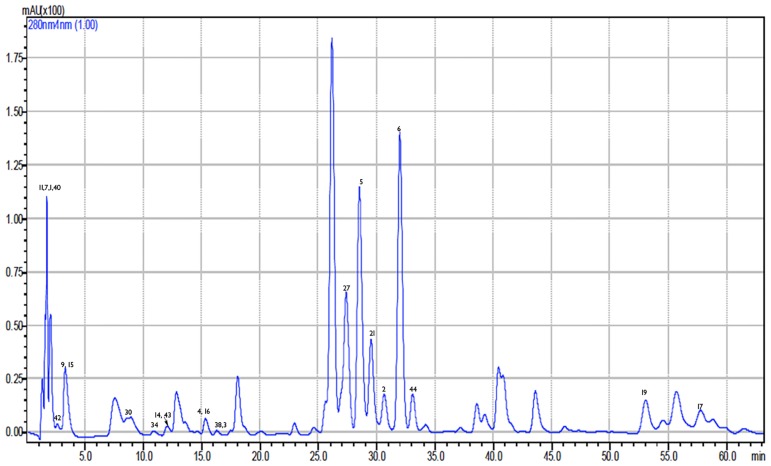
Fig 6. Magnified HPLC-UV chromatogram (at 280 nm) of Imathia propolis extract.
Fig 7. Distribution of chemical classes of compounds in propolis of different Greek regions.
Fig 8. Distribution of individual compounds in propolis of different Greek regions.
The results demonstrate that propolis sample from Imathia was of the most diverse regarding compounds identified and contained the highest concentrations (higher than the other studied samples).
Full Scan MS and PDA Putative Identification
Mass spectrometry data were also acquired in full scan mode (both negative and positive mode). Due to the chemistry of phenolic compounds, negative full scan mode was the most decisive tool in revealing the putative presence of several compounds not listed in the targeted screening approach. Several compounds were identified and are depicted in the respective Table (see Supplementary Material, “Table A in S1 File,”). To proceed to the tentative identification of substances, the literature on chemical constituents reported for propolis was considered. In particular, characteristic mass spectrometry ions-fragments for described compounds in the bibliography [22,34] were collected and compared to the fragment ions obtained in the current analyses. Elution order considering the chemical structures and subsequent polarity, and the characteristics of the chromatographic column used, have also been taken into account and paralleled to the bibliographical data.
GC-MS Analysis
Primary criterion in GC-MS analysis using full scan mode, is the computed match factor of the studied spectrum and the respective one of the library. The estimated non-polar retention index (n-alkane scale) was also used in parallel with respective literature values where applicable, and appear in Table 4. It is noteworthy that some compounds identified by HPLC-ESI-PDA/MS were also confirmed by GC-MS. Indicative examples are pinocembrin, naringenin, chrysin, techtochrysin, and others. The latter is not a “surprising finding” since GC analysis of those and similar constituents, has been reported by Christov and Bankova in 1992, using capillary GC and GC-ECD [38,39].
Table 4. Distribution of newly identified compounds (assessed by GC-MS) in ethanolic extracts of Greek propolis.
| RI Reference | RIL | RIE* | Retention Time (min) | Analyte | Nafplio | Amorgos | Crete | Kos | Imathia |
|---|---|---|---|---|---|---|---|---|---|
| - | no | 1536 | 12.22 | o-Orsellinaldehyde** | X | ||||
| - | no | 1564 | 19.91 | Cinnamylidene acetic acid | X | ||||
| [46] | 1969 | 1717 | 22.40 | Eudesmic acid | X | ||||
| - | no | 1694 | 24.82 | 3,8-dimethyl-4-(1-methylethylidene)-2,4,6,7,8,8a-hexahydro-5(1H)-azulenone | X | ||||
| [47] | 2700 | 2705 | 42.03 | Heptacosane | X | ||||
| [47] | 2800 | 2804 | 46.88 | Octacosane | X | ||||
| - | no | 2797 | 54.07 | (+)-Episesamin | X | ||||
| - | no | 3047 | 56.15 | 1-Octacosanol | X |
* RIE Estimated non-polar retention index (n-alkane scale), RIL literature non-polar retention index
** Confirmed by analytical standard
Several of the identified constituents such as cedrol, epicedrol, ferruginol, α-cadinol, have been reported as constituents of Greek propolis by several research groups (indicatively see [40]). Since, in this work, GC-MS analysis aimed only at the identification of new compounds the overall GC-MS fingerprint is not reported (however an obtained GC-MS chromatogram is presented, see above Fig 9, accompanied by indicative GC-MS mass spectra, “Figures A and B in S1 File”). GC-MS analysis of the samples revealed the existence of additional compounds, to our knowledge, not previously reported in Greek propolis samples (see the list of compounds in Table 4).
Fig 9. Magnified GC-MS chromatogram of Crete propolis sample.
Indicatively, first report of 3,8-dimethyl-4-(1-methylethylidene)-2,4,6,7,8,8a-hexahydro-5(1H)-azulenone is herein made (see Table 4, Amorgos extract). Azulene type compounds in Greek propolis have been reported only by Kalogeropoulos et al., namely, 1,2,3-triphenyl azulene was detected. Eudesmic acid found in Amorgos sample is an O-methylated trihydroxybenzoic acid that is reported in Eucalyptus spp. extracts [41]. 1-docosene, hexadecane, octacosane, hexacosene and heptacosane identified in Nafplio sample, are also reported for the first time in Greek propolis. Octacosane was reported as a constituent of Dubai propolis [42], while heptacosane and related compounds have been reported in the essential oil of Indian propolis [43]. Episesamin is a furofuran lignan that was reported by Bankova et al., in Canary Islands propolis [44]. o-Orsellinaldehyde, identified in Imathia sample, is a bioactive compound shown to exert cytotoxic effetcs against the human hepatoma hep B3 cells [45]. Consequently, the capacity of GC-MS to explore new constituents in natural products was verified in this work, unveiling eight new components of Greek propolis extracts.
Antioxidant Activity, TPC, and TFC
The concentration of the extract that results in 50% of scavenging on DPPH was defined as IC50. The latter was obtained from the linear regression equation of equation constructed from the concentrations of the sample extracts and the inhibition percentage of radical scavenging activity. Lower IC50 values corroborate greater radical scavenging and antioxidant activity. The Imathia propolis extract displayed the lowest IC50 value of 1.19 μg/mL, hence the most prominent antioxidant activity. All IC50 values and TPC, TFC values are presented in Table 5.
Table 5. Antioxidant activity, total phenolic and total flavonoids content of propolis extracts.
| Samples | Total Phenolic Content a,b | Total Flavonoids Content a,c | Scavenging Activity on DPPH IC50 (μg/mL)a | Normalized IC50 values (for PCA)d |
|---|---|---|---|---|
| Arkadia | 127.8±11.2 | 52.2±1.9 | 8.91±0.14 | 13.36 |
| Nafplio | 155.5±9.4 | 73.3±2.1 | 2.66±0.51 | 44.74 |
| Amorgos | 113.2±7.1 | 59.0±3.8 | 14.90±2.09 | 7.99 |
| Crete | 110.2±5.4 | 63.6±0.7 | 16.11±3.18 | 7.39 |
| Kos | 159.2±13.8 | 57.3±3.3 | 2.43±0.61 | 48.97 |
| Lakonia | 120.3±4.4 | 46.8±1.5 | 11.7±2.39 | 10.17 |
| Imathia | 181.0±7.8 | 86.0±2.9 | 1.19±0.29 | 100.00 |
| Kerkira | 144.2±10.5 | 52.1±0.7 | 7.46±0.33 | 15.95 |
| Quercetin | - | - | 0.46±0.09 |
a Values represent the mean of triplicate measurements ± standard deviation
b expressed as mg GAE/gdry extract
c Results in mg quercetin/gdry extract
d Normalization of IC50, setting value for Imathia at 100, calculating the rest accordingly.
Principal Component Analysis
The main statistical procedure followed in this work, was principal component analysis (PCA). It was employed in order to investigate possible relationships or groupings between propolis locations and the analysed compounds.
Two PCAs were applied and in both Varimax with Kaiser normalization method of rotation was selected. The first one involved the concentrations of all analytes as measured in the eight propolis locations. Seven principal components extracted by the analysis given that their eigenvalues exceed one (Kaiser’s rule). The first two components explained 50.8% of the total variance in initial values, while the following two accounted for 17.4% and 14.1% respectively (in total 82.3%). According to the rotated component matrix (“Table B in S1 File,” accompanied by the necessary correlation matrix “S3 File”) several analytes can be grouped having high (close to one) positive loadings in 1 to 7 principal components. In example, the first component is characterized by a high influence (loading>0.8) of Pinocembrin, Chrysin, Ferullic acid, Resveratrol, Naringenin, Pinobanksin-3o-acetate, Sakuranetin, Vitexin, Rosmarinic acid and Cynnamilidene acetic acid. Respectively, the second component is majorly driven by Pinostrobin, Kaempferol, Kaempferide, Acacetin, Isosakuranetin, Myricetin, and Ursolic acid while the third one is mainly related to Galangin, p-Coumaric acid, Pinobanksin, Caffeic acid, Daidzein and Genistein (Fig 10).
Fig 10. First three component plot in rotated space.
The component scores of the propolis locations revealed no further grouping between sites, as each of them presented high values in distinct components. According to the analysis’ results, Imathia, Lakonia and Kos succeeded high scores in component 1, 2 and 3 respectively (Fig 11), which revealed an interrelation of those locations with the propolis compounds which demonstrate high influence on component 1, 2 and 3 as aforementioned.
Fig 11. Plot of location’s scores in first three principal components.
The second PCA regarded three classes of compounds, DPPH, TPC, and yet the eight propolis locations (see respective Figs 12 and 13). Three major classes of compounds, namely flavonoids, benzoic acid and cinnamic acid derivatives were selected. The selection was based on their occurrence in the studied propolis samples. For each class the sum of concentrations of individual components were calculated, for each propolis sample-location.
Fig 12. Component plot in rotated space.
Fig 13. Plot of location’s scores in two principal components.
In this analysis, the principal components extracted, according to Kaiser’s rule which retains only factors with eigenvalues that exceed one, were two which cumulatively explained 91.1% of the variance in the initial data. Further, component loadings in the rotated space clearly revealed two groups (see Fig 12). The first one comprising of Flavonoids, DPPH, and TPC has high positive loadings in component 1 while the Cinnamic—Benzoic acid group most influences component 2 (the correlation matrix between variables, and component loadings after varimax rotation are presented in supplementary material “Tables C and D in S1 File” correspondingly).
This principal component analysis was consistent with the first one in highlighting Imathia and Kos as two locations having a unique behaviour. Indeed, Imathia performed a high score in component 1 demonstrating that Flavonoids, DPPH, and TPC are strongly related to its propolis compounds. On the other hand, Kos’s propolis is correlated with the Cinnamic—Benzoic acid group.
Discussion
Analytical Method and Results
The main aim of this work was the development of a novel analytical method for the identification and quantification of several phenolic compounds in raw propolis. Considering that these compounds are found in several plants and products, this method can serve as a universal tool in phytochemical analysis. For the identification and quantification of compounds, an appropriate extraction protocol should be applied with satisfactory extraction efficiency. Concerning extraction of compounds from raw propolis is known that several solvents are used in efforts to explore its chemical profile, and the different constituents or variations of them depending on the solvent or combinations of solvents used. When liquid chromatographic analysis is employed, the majority of propolis’ published works report ethanol or methanol or respective mixtures with water (where alcohol predominates) as the extraction solvent. Consequently, in the presented work an ethanol: water mixture (4:1) was selected for the extraction. Other solvents such as ethyl acetate, or dichloromethane were used in this work on a pilot basis, but did not show substantial differences in terms of quantities and number of compounds extracted, hence not further investigated.
The targeted analysis presented in this work has disclosed several new active substances, never reported in Greek propolis. Regions for which previous reports for propolis samples exist, and were also considered in this report, were Kos, Lakonia, Crete, Arkadia, and Kerkira. To evaluate and interpret results, it is worth to mention, that the sampling location (within a particular region) seems to detrimentally interplay in the chemical composition of samples. Therefore, variations between research works conducted within specific regions are expected to occur since it is unlike to refer in all studies, to the exact identical location. These variations are reflected by the possible discrepancies in terms of compounds detected, and respective concentrations. Another factor that should be regarded is that bees tend to select plants that are resin donors and possible year variations regarding such plants would impact their foraging preference. Last but not least, season in which propolis is collected by the bees is also a key determinant factor for its composition. Consequently, any differences observed, reflect to an extent, the differential foraging activity of bees within a specific region and period of time. Based on the above considerations, the incidence of the newly identified compounds in Greek regions also studied in the past are presented in Table 6.
Table 6. New compounds identified by HPLC-PDA-ESI/MS, in Greek regions (also investigated in the past).
| Location | Kos* | Arkadia*,** | Lakonia* | Crete*,** | Kerkira*** |
|---|---|---|---|---|---|
| Compounds | p-coumaric acid | PIN-7ME | CAPE | Apigenin | Kaempferol |
| Caffeic acid | CAPE | Acacetin | Galangin | Rutin | |
| CAPE | Syringic acid | Myricetin | Isorhamnetin | Vanillin | |
| Apigenin | Pinostrobin | Pinostrobin | Rhamnetin | Rosmarinic acid | |
| Pinocembrin | Isorhamnetin | Kaempferide | CAPE | ||
| Eriodictyol | Eriodictyol | Luteolin | |||
| Kaempferol | Hesperetin | ||||
| Galangin | |||||
| Pinobanksin | |||||
| Hesperetin |
* Graikou et al. 2016,
** Kalogeropoulos et al. 2009,
*** Çelemli et al. 2013
Bioactivities of Constituents
In this paper, a brief overview of the bioactivities demonstrated by newly identified constituents of Greek propolis is provided. Hence, many of the new compounds, exhibit substantial activity as components of active extracts or as separately studied compounds. Indicatively, 1-octacosanol is a long-chain aliphatic alcohol that was stated to exhibit antio-angiogenic activity [48] and antioxidant activity [49]. With regard to azulene derivatives, extracts or natural products (such as chamomile tea) containing them (such as the one detected in Amorgos sample) are known for the significant bioactivity that they exhibit (indicatively see [50]).
(+)-Episesamin detected in Cretan sample, was found to exert antineoplastic effects in human hepatocellular carcinoma cell lines [51] and anti-inflammatory effects via inhibition of adipogenesis [52]. Eudesmic acid was found as a constituent of methanol extract of Abutilon Indicum leaves. The latter demonstrated moderate antibacterial activity [53]. Cinnamylidene acetic acid is usually used as a building block for the construction of bioactive compounds [54].
In this work, the first report of rosmarinic acid in Greek propolis is made (Kerkira sample). Rosmarinic acid is a constituent of several aromatic plants [55] and has also displayed antimutagenic activity as this was evaluated by the micronucleus assay in mice [56]. Rosemary extracts, rich in rosmarinic acid, also demonstrated antioxidant and antimicrobial properties [57]. Hesperetin is a plant bioflavonoid that is abundant in citrus fruits [58]. It exerts significant pharmacological properties, such as antioxidant and anti-inflammatory properties. Isorhamnetin a flavonol that was also detected exhibits significant bioactivity. More specifically, isorhamnetin was reported to show cytotoxic effects on human colon cancer cells [59]. Isosakuranetin is reported in propolis samples and in several plants belonging to divergent botanical families [60]. In addition, it was reported to have the potential to act as a protective agent for skin photoaging [61].
Other known constituents that were identified, are also bioactive and contribute to the bioactive profile of propolis. Indicatively, ferruginol (identified by GC-MS) is an abietane diterpene that along with several of its derivatives, in ethanolic extracts displayed cytotoxic activity against human tumor cells [62].
In this regard, first reports of several compounds in Greek propolis, highlight the chemical diversity of Greek propolis and postulate that revisiting natural products can lead to exploration of news constituents with pronounced biological activity, opening new frontiers to their exploitation.
Biosynthetic Pathways of Newly Detected Compounds
In this section, a brief reference to biosynthetic pathways that might lead to the formation of several compounds identified in this work will be provided. Flavonoids (referred also as bioflavonoids), which is the major category of compounds determined in this work, are secondary metabolic products, therefore they have no straightforward implication with the development of plants. Flavonoids’ precursor molecule is phenylalanine. The latter is deaminated-transformed to cinnamic acid by the enzyme, phenylalanine ammonia lyase [63]. With regard to the newly identified flavonoids, acacetin is an aglycone that can be derived from hydrolysis of the respective flavonoid glycoside from the leaves of some plants such as Robinia Pseudacacia [64]. Kaempferide that is the 4’-O-methyl derivative of kaempferol was detected in one out of the eight samples implying that kaempferol, as a plant constituent, might have undergone the respective methylation. Kaempferide was reported as propolis constituent in Italian, Ukranian and FYROM propolis in a 2008 work [65], albeit is usually reported as a component of Brazilian propolis (indicatively see [35]). Sakuranetin is biosynthesized from naringenin, via the action of two agents, the S-adenosyl-L-methionine influenced by the enzyme naringenin-7-o-methyltransferase [66, 67]. Lately, sakuranetin was detected in Greek propolis (Graikou et al. 2016, in 3 regions Euboia, Evros, and Chalkidiki), but in this work, this compound is detected in a new studied region of Greece (Imathia). Isosakuranetin is also reported to be biosynthesized from naringenin via an O-methyltransferase enzyme expressed in E. coli [68]. Luteolin’s biosynthesis in Rosmarinus officinalis has been postulated by del Bano et al. [69]. In this work, naringenin was proposed to be hydroxylated to eriodictyol and finally converted to luteolin by the enzyme flavone synthase. Eriodictyol an aglyconic compound that was detected in Nafplio, Kos and Imathia propolis is a biotransformation product of naringenin, and hence, under specific conditions, it can be detected in propolis. Notwithstanding, naringenin was detected in all samples except Kos sample. Lin et al., reported pinostrobin (a flavanone glycoside), as a constituent of a 70% acetonic fraction of Viscum angulatum stems [70].
Antioxidant Activity
Diphenylpicrylhydrazyl (DPPH) radical scavenging activity assay was implemented and assessed for all studied samples, exhibiting robust performance. Numerous groups have reported the use of the stable free radical DPPH for estimating antioxidant activity for several plant extracts and natural products. DPPH is considered as a stable free radical by virtue of the delocalization of the spare electron over the molecule as a whole so that the molecules do not form dimers, like most other free radicals. In the same context, the order of DPPH radical scavenging activity is in concordance with the total phenolic content, corroborating that antioxidant activity increased (lower IC50 values) with the increase of TPC.
With regard to the profound activity of Imathia propolis extract, the latter seems to be attributed to the high concentration of flavonoids that was portrayed both from targeted chemical analysis and TFC measurements. Imathia has one of the most fertile plains in Greece, listed along with Pella region, key areas of cultivation of peaches (an important crop for Greece with an export quantity of 155263 tones for 2012 based on FAO statistics, [71]). Nevertheless, peaches are bibliographically reported as a profound source of flavonoids [72] and as notable producers of both nectar and pollen that constitute them attractive to bees [73]. In the expected flight range of bees for the particular location that hives were positioned, several attractive crops, aromatic and edible wild plants are also abundant. In this context, apart from predominant peach trees, cotton trees, raspberries, chamomile, mentha, lavender, oregano are frequently found [74]. For Kos sample (the second most active propolis extract), it should be noted, that the sample originated from the North-East part of the Island that is the most prolific part with widespread cultivations of fruit trees (orange, mandarin, peach trees), and plants such as lavender, thyme, heather, sage and others that render this region an ideal area for foraging. On the other hand, although Crete is known for its diverse flora and abundance of fruit trees, the specific propolis extract displayed the lowest antioxidant activity. The latter is in line with the report of the Cretan beekeeper that had his hive positioned in a relatively isolated area targeting specific crops, a fact reflected in chemical analysis as well. Nonetheless, the explicit description of the flora of each studied region was not pursued since it was relatively difficult to monitor foraging bees and conclude to the most popular plants-flowers that they visited.
With regard to PCA, although it was not the primary aim of this work, it disclosed certain correlations that can be used as a basis for future studies. Such pursued works can underscore a more extensive sampling scheme within regions, and the addition of other antioxidant assays that will strengthen PCA with the inclusion of interrelated additional variables. The latter may unveil other correlations among components and the flora of the studied areas.
Conclusions
The developed analytical method can serve as a universal tool to detect and identify common phenolic compounds that are not only encountered in propolis but also in other plants and their extracts. Hence, it has broad applicability and can be implemented in several projects that aim to elucidate chemical composition of beehive products, and other natural products and their extracts. A further step to improve this work, will be the adaptation of this approach to a high-resolution mass spectrometry scheme circumventing the issue of analytical standards availability and proceed to fingerprinting analysis via untagreted analysis of propolis samples elucidating all present compounds. Concerning the antioxidant activity of propolis extracts, a pronounced activity was evidenced for some of the extracts, with two of them displaying IC50 values below 2.5 μM, comparably active to that of quercetin. The demonstrated activity along with the unveiling of new substances present the necessity to explore propolis and other apiculture products continuously. Last but not least, targeted bioassays are currently underway, to further explore the bioactivity of these extracts.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank all beekeepers fot the kind provision of propolis samples (and Dr. Alissandrakis for the Cretan sample) for the scope of this work.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bankova V. Recent trends and important developments in propolis research. Evidence-based complementary and alternative medicine: eCAM. 2005;2(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bankova V. Chemical diversity of propolis and the problem of standardization. Journal of ethnopharmacology. 2005;100(1–2):114–7. 10.1016/j.jep.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 3.Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116(2):452–61. [Google Scholar]
- 4.de Figueiredoa SM, Nogueira-Machado JA, Almeida Bde M, Abreu SRL, de Abreu JAS, Filho SAV, et al. Immunomodulatory Properties of Green Propolis. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery. 2014;8:85–94. [DOI] [PubMed] [Google Scholar]
- 5.Lofty M. Biological Activity of Bee Propolis in Health and Disease. Asian Pacific Journal of Cancer Prevention. 2006;7:22–31. [PubMed] [Google Scholar]
- 6.Benhanifia M, Mohamed WM. Phenolics Constituents of Different Types of Propolis and their Antimicrobial Activities. Anti-Infective Agents. 2015;13(1):17–27. [Google Scholar]
- 7.Varoni EM, Lodi G, Sardella A, Carrassi A, Iriti M. Plant polyphenols and oral health: old phytochemicals for new fields. Current medicinal chemistry. 2012;19(11):1706–20. [DOI] [PubMed] [Google Scholar]
- 8.Calderon-Montano JM, Burgos-Moron E, Perez-Guerrero C, Lopez-Lazaro M. A review on the dietary flavonoid kaempferol. Mini reviews in medicinal chemistry. 2011;11(4):298–344. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Lazaro M. Distribution and biological activities of the flavonoid luteolin. Mini reviews in medicinal chemistry. 2009;9(1):31–59. [DOI] [PubMed] [Google Scholar]
- 10.Sun LP, Chen AL, Hung HC, Chien YH, Huang JS, Huang CY, et al. Chrysin: a histone deacetylase 8 inhibitor with anticancer activity and a suitable candidate for the standardization of Chinese propolis. J Agric Food Chem. 2012;60(47):11748–58. 10.1021/jf303261r [DOI] [PubMed] [Google Scholar]
- 11.Jnawali HN, Lee E, Jeong KW, Shin A, Heo YS, Kim Y. Anti-inflammatory activity of rhamnetin and a model of its binding to c-Jun NH2-terminal kinase 1 and p38 MAPK. Journal of natural products. 2014;77(2):258–63. 10.1021/np400803n [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Luo Q, Bi J, Ding J, Ge S, Chen F. Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chemico-biological interactions. 2014;224C:149–56. [DOI] [PubMed] [Google Scholar]
- 13.Abdelwahab SI, Mohan S, Abdulla MA, Sukari MA, Abdul AB, Taha MM, et al. The methanolic extract of Boesenbergia rotunda (L.) Mansf. and its major compound pinostrobin induces anti-ulcerogenic property in vivo: possible involvement of indirect antioxidant action. Journal of ethnopharmacology. 2011;137(2):963–70. 10.1016/j.jep.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Coppo E, Marchese A. Antibacterial Activity of Polyphenols. Curr Pharm Biotechno. 2014;15(4):380–90. [DOI] [PubMed] [Google Scholar]
- 15.Saad MA, Abdel Salam RM, Kenawy SA, Attia AS. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia reperfusion. Pharmacological reports: PR. 2015;67(1):115–22. 10.1016/j.pharep.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 16.Omene CO, Wu J, Frenkel K. Caffeic Acid Phenethyl Ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investigational new drugs. 2012;30(4):1279–88. 10.1007/s10637-011-9667-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Omene C, Karkoszka J, Bosland M, Eckard J, Klein CB, et al. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011;308(1):43–53. 10.1016/j.canlet.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordo J, Maximo P, Cabrita E, Lourenco A, Oliva A, Almeida J, et al. Thymus mastichina: chemical constituents and their anti-cancer activity. Natural product communications. 2012;7(11):1491–4. [PubMed] [Google Scholar]
- 19.Kancheva VD, Kasaikina OT. Bio-antioxidants—a chemical base of their antioxidant activity and beneficial effect on human health. Current medicinal chemistry. 2013;20(37):4784–805. [DOI] [PubMed] [Google Scholar]
- 20.Graikou K, Popova M, Gortzi O, Bankova V, Chinou I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? Lwt-Food Sci Technol. 2016;65:261–7. [Google Scholar]
- 21.Lagouri V, Prasianaki D, Krysta F. Antioxidant Properties and Phenolic Composition of Greek Propolis Extracts. Int J Food Prop. 2014;17(3):511–22. [Google Scholar]
- 22.Falcao SI, Vale N, Gomes P, Domingues MR, Freire C, Cardoso SM, et al. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: uncommon propolis rich in flavonoid glycosides. Phytochemical analysis: PCA. 2013;24(4):309–18. 10.1002/pca.2412 [DOI] [PubMed] [Google Scholar]
- 23.Chauzat MP, Cauquil L, Roy L, Franco S, Hendrikx P, Ribiere-Chabert M. Demographics of the European apicultural industry. PloS one. 2013;8(11):e79018 10.1371/journal.pone.0079018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Dong Y, Du H, Shi H, Peng Y, Li X. Antioxidant compounds from propolis collected in Anhui, China. Molecules. 2011;16(4):3444–55. 10.3390/molecules16043444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ICH. VALIDATION OF ANALYTICAL PROCEDURES: TEXT AND METHODOLOGY Q2(R1) http://wwwichorg/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guidelinepdf.
- 26.SANCO/12571/2013. Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed.:http://ec.europa.eu/food/plant/pesticides/guidance_documents/docs/qualcontrol_en.pdf, accessed 10 Dec 2015.
- 27.Betz JM, Brown PN, Roman MC. Accuracy, precision, and reliability of chemical measurements in natural products research. Fitoterapia. 2011;82(1):44–52. 10.1016/j.fitote.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo MR, Iditoiu C, Chambre D, Lupea AX. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem Pap. 2007;61(3):214–6. [Google Scholar]
- 29.Yang HS, Dong YQ, Du HJ, Shi HM, Peng YH, Li XB. Antioxidant Compounds from Propolis Collected in Anhui, China. Molecules. 2011;16(4):3444–55. 10.3390/molecules16043444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol. 2009;47(8):1848–51. 10.1016/j.fct.2009.04.040 [DOI] [PubMed] [Google Scholar]
- 31.Shukla S, Mehta A, Bajpai VK, Shukla S. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem Toxicol. 2009;47(9):2338–43. 10.1016/j.fct.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 32.Madaan R, Bansal G, Kumar S, Sharma A. Estimation of Total Phenols and Flavonoids in Extracts of Actaea spicata Roots and Antioxidant Activity Studies. Indian J Pharm Sci. 2011;73(6):666–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd LE. Spectrophotometric determination of quercetin. Analytical Chemistry. 1959;31(7):1184–7. [Google Scholar]
- 34.Falcao SI, Vilas-Boas M, Estevinho LM, Barros C, Domingues MR, Cardoso SM. Phenolic characterization of Northeast Portuguese propolis: usual and unusual compounds. Analytical and bioanalytical chemistry. 2010;396(2):887–97. 10.1007/s00216-009-3232-8 [DOI] [PubMed] [Google Scholar]
- 35.Szliszka E, Kucharska AZ, Sokol-Letowska A, Mertas A, Czuba ZP, Krol W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evidence-based complementary and alternative medicine: eCAM. 2013;2013:976415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Economou A, Botitsi H, Antoniou S, Tsipi D. Determination of multi-class pesticides in wines by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Journal of chromatography A. 2009;1216(31):5856–67. 10.1016/j.chroma.2009.06.031 [DOI] [PubMed] [Google Scholar]
- 37.Medic-Saric M, Bojic M, Rastija V, Cvek J. Polyphenolic Profiling of Croatian Propolis and Wine. Food Technol Biotech. 2013;51(2):159–70. [Google Scholar]
- 38.Bankova V, Christov R, Stoev G, Popov S. Determination of Phenolics from Propolis by Capillary Gas-Chromatography. J Chromatogr. 1992;607(1):150–3. [Google Scholar]
- 39.Christov R, Bankova V. Gas-Chromatographic Analysis of Underivatized Phenolic Constituents from Propolis Using an Electron-Capture Detector. J Chromatogr. 1992;623(1):182–5. [Google Scholar]
- 40.Melliou E, Stratis E, Chinou I. Volatile constituents of propolis from various regions of Greece—Antimicrobial activity. Food Chem. 2007;103:375–80. [Google Scholar]
- 41.Conde E, Cadahia E, Garcia-Vallejo MC. HPLC analysis of flavonoids and phenolic acids and aldehydes in Eucalyptus spp. Chromatographia. 1995;41(11/12):657–60. [Google Scholar]
- 42.Said SA, Khan SA, Ahmad I, Ali HS. Chemical composition of Egyptian and UAE propolis. Pakistan Journal of Pharmaceutical Sciences. 2006;19(1):58–61. [PubMed] [Google Scholar]
- 43.Naik DG, Vaidya HS, Namjoshi TP. Essential oil of Indian propolis: chemical composition and repellency against the honeybee Apis florea. Chemistry & biodiversity. 2013;10(4):649–57. [DOI] [PubMed] [Google Scholar]
- 44.Bankova VS, Christov RS, Tejera AD. Lignans and other constituents of propolis from the Canary islands. Phytochemistry. 1998;49(5):1411–5. [Google Scholar]
- 45.Lin JT, Liu WH. o-Orsellinaldehyde from the submerged culture of the edible mushroom Grifola frondosa exhibits selective cytotoxic effect against Hep 3B cells through apoptosis. J Agric Food Chem. 2006;54(20):7564–9. 10.1021/jf0616762 [DOI] [PubMed] [Google Scholar]
- 46.Yayli N, Gulec C, Ucuncu O, Yasar A, Ulker S, Coskuncelebi K, et al. Composition and antimicrobial activities of volatile components of Minuartia meyeri. Turk J Chem. 2006;30(1):71–6. [Google Scholar]
- 47.von Kovats E. 206. Gas chromatographische Charakterrisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helvetica Chimica Acta. 1958;41(7):1915–32. [Google Scholar]
- 48.Thippeswamy G, Sheela ML, Salimath BP. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF-<kappa>B and its DNA binding activity. European journal of pharmacology. 2008;588(2–3):141–50. 10.1016/j.ejphar.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 49.Firdous S, Khan K, Zikr-Ur-Rehman S, Ali Z, Soomro S, Ahmad VU, et al. Isolation of phytochemicals from Cordia rothii (Boraginaceae) and evaluation of their immunomodulatory properties. Records of Natural Products. 2014;8:51–5. [Google Scholar]
- 50.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytotherapy research: PTR. 2006;20(7):519–30. 10.1002/ptr.1900 [DOI] [PubMed] [Google Scholar]
- 51.Freise C, Trowitzsch-Kienast W, Ruehl M, Erben U, Seehofer D, Kim KY, et al. (+)-Episesamin exerts anti-neoplastic effects in human hepatocellular carcinoma cell lines via suppression of nuclear factor-kappa B and inhibition of MMP-9. Investigational new drugs. 2012;30(6):2087–95. 10.1007/s10637-011-9762-x [DOI] [PubMed] [Google Scholar]
- 52.Freise C, Trowitzsch-Kienast W, Erben U, Seehofer D, Kim KY, Zeitz M, et al. (+)-Episesamin inhibits adipogenesis and exerts anti-inflammatory effects in 3T3-L1 (pre)adipocytes by sustained Wnt signaling, down-regulation of PPARgamma and induction of iNOS. The Journal of nutritional biochemistry. 2013;24(3):550–5. 10.1016/j.jnutbio.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 53.Rajput AP, Patel MK. Chemical investigation and biological activity of phytoconstituents from methanol extract of Abutilon indicum leaves. Journal of Chemical and Pharmaceutical Research. 2012;4(8):3959–65. [Google Scholar]
- 54.Hardej D, Ashby CR Jr., Khadtare NS, Kulkarni SS, Singh S, Talele TT. The synthesis of phenylalanine-derived C5-substituted rhodanines and their activity against selected methicillin-resistant Staphylococcus aureus (MRSA) strains. European journal of medicinal chemistry. 2010;45(12):5827–32. 10.1016/j.ejmech.2010.09.045 [DOI] [PubMed] [Google Scholar]
- 55.Miron TL, Plaza M, Bahrim G, Ibanez E, Herrero M. Chemical composition of bioactive pressurized extracts of Romanian aromatic plants. Journal of Chromatography A. 2011;1218:4918–27. 10.1016/j.chroma.2010.11.055 [DOI] [PubMed] [Google Scholar]
- 56.Furtado MA, de Almeida LCF, Furtado RA, Cunha WR, Tavares DC. Antimutagenicity of rosmarinic acid in Swiss mice evaluated by the micronucleus assay. Mutation research. 2008;657:150–4. 10.1016/j.mrgentox.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 57.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radical Research. 2006;40(2):223–31. 10.1080/10715760500473834 [DOI] [PubMed] [Google Scholar]
- 58.Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid composition of Citrus juices. Molecules. 2007;12(8):1641–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaramillo S, Lopez S, Varela LM, Rodriguez-Arcos R, Jimenez A, Abia R, et al. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J Agric Food Chem. 2010;58(20):10869–75. 10.1021/jf102669p [DOI] [PubMed] [Google Scholar]
- 60.Guimaraes NS, Mello JC, Paiva JS, Bueno PC, Berretta AA, Torquato RJ, et al. Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem Toxicol. 2012;50(3–4):1091–7. 10.1016/j.fct.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 61.Jung H, Lee EH, Lee TH, Cho MH. The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging. International journal of molecular sciences. 2016;17(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S, Wang P, Deng G, Yuan W, Su Z. Cytotoxic compounds from invasive giant salvinia (Salvinia molesta) against human tumor cells. Bioorganic & medicinal chemistry letters. 2013;23(24):6682–7. [DOI] [PubMed] [Google Scholar]
- 63.Tan EC, Karsani SA, Foo GT, Wong SM, Rahman NA, Khalid N, et al. Proteomic analysis of cell suspension cultures of Boesenbergia rotunda induced by phenylalanine: identification of proteins involved in flavonoid and phenylpropanoid biosynthesis pathways. Plant Cell Tiss Organ Cult. 2012;111:219–29. [Google Scholar]
- 64.Ebel J, Barz W, Grisebach H. Biosynthesis of acacetin in Robinia Pseudacacia: incorporation of multiple labelled p-methoxycinnamic acid. Phytochemistry. 1970;9:1529–34. [Google Scholar]
- 65.Medana C, Carbone F, Aigotti R, Appendino G, Baiocchi C. Selective analysis of phenolic compounds in propolis by HPLC-MS/MS. Phytochemical analysis: PCA. 2008;19(1):32–9. 10.1002/pca.1010 [DOI] [PubMed] [Google Scholar]
- 66.Shimizu T, Lin F, Hasegawa M, Nojiri H, Yamane H, Okada K. The potential bioproduction of the pharmaceutical agent sakuranetin, a flavonoid phytoalexin in rice. Bioengineered. 2012;3(6):352–7. 10.4161/bioe.21546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu T, Lin F, Hasegawa M, Okada K, Nojiri H, Yamane H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. The Journal of biological chemistry. 2012;287(23):19315–25. 10.1074/jbc.M112.351270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim DH, Kim BG, Lee Y, Ryu JY, Lim Y, Hur HG, et al. Regiospecific methylation of naringenin to ponciretin by soybean O-methyltransferase expressed in Escherichia coli. J Biotechnol. 2005;119(2):155–62. 10.1016/j.jbiotec.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 69.del Bano MJ, Lorente J, Castillo J, Benavente-Garcia O, Marin MP, Del Rio JA, et al. Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. postulation of a biosynthetic pathway. J Agric Food Chem. 2004;52(16):4987–92. 10.1021/jf040078p [DOI] [PubMed] [Google Scholar]
- 70.Lin JH, Chiou YN, Lin YL. Phenolic glycosides from Viscum angulatum. Journal of natural products. 2002;65(5):638–40. [DOI] [PubMed] [Google Scholar]
- 71.FAO. FAO STATISTICS 2012 [http://www.fao.org/faostat/en/-data/TP.
- 72.Cocconi E, Stingone C, Zanotti A, Trifiro A. Characterization of polyphenols in apricot and peach purees by UHPLC coupled to HRMS Q-Exactive() mass spectrometer: an approach in the identification of adulterations. J Mass Spectrom. 2016;51(9):742–9. 10.1002/jms.3818 [DOI] [PubMed] [Google Scholar]
- 73.Benedek P, Nyeki J. Studies on the bee pollination of peach and nectarine. Acta Horticulturae. 1996;374:169–76. [Google Scholar]
- 74.GAIAPEDIA. http://www.gaiapedia.gr/gaiapedia/index.php.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.