Abstract
The effect of age on male Glossina fuscipes fuscipes, Newstead, and Glossina palpalis palpalis, Austin (Diptera: Glossinidae) competiveness were investigated with a view to estimate optimal age for sterile male release. Sterile insect technique involves the mass production, sterilization and sequential release of males of the target species to out compete the wild male population. Mating between released sterile males and wild females produce inviable progeny and the population is reduced over several generations to unsustainable levels. It is vital that the released male are of high quality and are sexually competitive. Age is one parameter affecting the sexual competiveness of the male tsetse fly. The optimal release age was estimated by assessing sexual competitiveness of flies of different age categories, 1, 5, 8 and 13-days after adult eclosion. A walk-in field-cage was used in order to approximate as closely as possible the actual field scenario during sterile insect release programes. It was shown that 8 and 13-day old males mated significantly more frequently, i.e. were more competitive, in the presence of equal numbers of 1 and 5-day old males. The age of male tsetse flies significantly affected competitiveness in both species studied. The ability of G. f. fuscipes to inseminate was not age dependent, and insemination occurred in all females that mated regardless of male age. In G. p. palpalis, however, 1-day old males were least able to inseminate. Mating duration was not significantly affected by age in both species. Eight to thirteen day old males of the test species are here recommended as the optimal sterile male release age.
Résumé
L'effet de l'âge sur la compétitivité des mâles de Glossina fuscipes fuscipes et de Glossina palpalis palpalis a été étudié en vue de déterminer l'âge optimal pour le lâcher de mâles stériles. La technique de l'insecte stérile (TIS) consiste en une production de masse, en la stérilisation et au lâchage de mâles de l'espèce cible afin qu'ils competissent avec la population de mâles sauvages. L'accouplement entre mâles stériles lâches et femelles sauvages ne produit pas de progéniture, ce qui conduit au bout de plusieurs générations à une réduction de la population à un niveau non sur vivable. Il est primordial que les mâles lâches soient de bonne qualité et sexuellement compétitifs. L'âge est l'un des paramètres affectant la compétitivité des mouches tsetse mâles. Il était donc nécessaire d'estimer l'âge optimal pour lâcher des mâles en comparant la compétitivité de différentes catégories d́âges (1, 5, 8 et 13 jours après leur émergence). La méthode dite du « field-cage » a été utilisée afin d'étudier le comportement des mâles TIS dans les conditions aussi proches que possible de la réalité. Il a été démontré que les mâles de 8 et 13 jours s'accouplent plus fréquemment que les mâles de 1 a 5 jours. Pour les deux espèces étudiées, l'âge affecte significativement la compétitivité des tsetse mâles. La capacité des mâles de G. f. fuscipes à inséminer n'est pas fonction de l'âge ; toutes les femelles accouplées sont inséminées. Chez G. p. palpalis cependant, les mâles de 1 jours sont les moins inséminées. La durée de l'accouplement n'est pas significativement affectée par l'âge dans les deux espèces. Les mâles de 8 et 13 jours des deux espèces testées sont les plus recommandés pour le lâcher des mâles stériles.
Keywords: optimal mating age, male competitiveness, sterile insect technique, Glossina fuscipes fuscipes, Glossina palpalis palpalis
Keywords: âge optimale d'accouplement, compétitivité des mâles, technique de l'insecte stérile, Glossina fuscipes fuscipes, Glossina palpalis palpalis
Introduction
Mating behavior in tsetse flies (Glossina) has been the focus of recent research (Mutika et. al., 2001; Olet et. al., 2002; Carlson and Schlein, 1991) because of its implications in the efficiency of the sterile male insect technique as a control strategy (Knipling, 1955; 1959; 1963). Tsetse cause significant economic loss by transmitting both human and animal African trypanosomosis; a disease caused by protozoan blood parasites of the genus Trypanosoma. It is estimated that annual losses caused by animal trypanosomosis in agricultural production in Sub-Saharan Africa are in the order of UK £ 3 billion and more than 100 human lives a day (Budd, 1999).
Vector control has been one option in the control of both human and animal forms of the disease. Tsetse control methods have evolved from game and bush clearing at the beginning of the 20th century to insecticide spraying in the 1950s (Allsopp, 2001), and more recently traps, targets (Brightwell et. al., 1997) and live bait technologies have been used to control tsetse (Thomson and Wilson 1991). These methods have been used successfully with dramatic reductions in tsetse population sizes (Leak, 1999). However, in the absence of sustained control efforts and protection of cleared areas, tsetse fly populations have recovered from residual pockets and/or re-invaded from neighbouring territories (Brightwell et. al., 1997).
The area-wide-approach to tsetse control aims at control of entire populations and to effectively prevent re-invasion or recovery from residual pockets. The sterile insect technique is one way in which this can be achieved (Knipling, 1963). The sterile insect technique relies on the release of mass reared, sexually sterilized males and is environmentally benign. Mating between released sterile males and wild females results in embryonic arrest followed by expulsion of the dead embryo (Van der Vloedt, et. al., 1978). Successful area-wide use of sterile insect programs have been implemented against insect pests and disease vectors such as the Mediterranean fruit fly, Ceratitis capitata (Hendriches et. al., 1995), the New World screw-worm, Cochliomyia hominivorax (Lindquist et. al., 1992), and the mosquito, Culex quinquefasciatus, the vector of bancroftian filariasis (Patterson et. al., 1977; Curtis and Andreasen, 2000). More recently, Vreysen et. al. (2000), have demonstrated the application of the use of sterile insect techniques in the area-wide eradication of Glossina austeni and elimination of animal trypanosomosis transmission on Ungunja Island, Zanzibar (Dyck et. al., 2000). This case, in addition to earlier successful attempts in Burkina Faso (Politzar and Cuisance, 1984) and Nigeria (Oladunmade, et. al., 1990), have inspired a continental strategy to progressively reduce isolated tsetse populations to unsustainable numbers. This undertaking will require mass production of sterile males for sustained sequential release until significant population reductions are achieved (Feldmann and Hendrichs, 2001).
The labor and attendant costs of producing sufficient numbers of sterile male tsetse flies for release inevitably requires that the mating success and competitiveness of the released flies are optimal (Mutika, et. al. 2001; Alphey and Andreason, 2002). Olet, et. al. (2002) investigated sexual receptivity and age in G. pallidipes and showed that older males copulated more frequently than younger ones. Releasing sterile males of an optimal age would improve efficiency of the sterile males to out compete the wild males. This will contribute towards the efficiency and reduce costs of a release program. It is therefore vital to investigate the most succesful age for copulation and sperm transfer in male tsetse flies, with a view to establish the optimal age for sterile male release for each species. Earlier investigations on the age and sexual competitiveness of tsetse have been done in fly holding cages that confined the flies to unnaturally small and restricted space. Here we use a walk-in fieldcage (Calkins and Webb, 1983; Mutika, 2001) to investigate the effect of age on the mating competitiveness of the test species; Glossina fuscipes fuscipes Austen, and Glossina palpalis palpalis Austen. Information on optimal sterile male release age, duration of copulation, insemination rates, and ambient environmental conditions of the experiments are reported.
Materials and Methods
The tsetse flies
The G. f. fuscipes colony used in this study were first colonized in 1986, while the G. p. palpalis dated from 1981 and have been in culture for 64 and 88 generations respectively. Both colonies were maintained in vitro on silicone membrane with bovine blood diet, at the FAO/IAEA Agriculture and Biotechnology Laboratory at Seibersdorf, Austria. The flies were kept under standard colony conditions of pure bovine blood diet. The flies were offered a blood meal everyday except on Sundays. Holding temperature was maintained at 24 ± 1 °C, and 70 to 80% relative humidity (Feldmann, 1994a,b). The males were chilled at 4 °C and color marked using Rowny® acrylic paint one day before the experiments. All female flies used in experiments were 8 days old which is when female receptivity to males was determined to be maximal (Olet et al., 2002). Males are capable of inseminating at emergence because of their well-developed accessory glands (Forster, 1976). In both colonies of the test species that were used in this study, the males were able to mount and inseminate successfully on the first day after eclosion, in the absence of older males.
The field-cage
The cage used was the same as that described by Calkins and Webb (1983). The cage netting forms a cylinder of 2.9 m diameter and 2.0 m height. The entrance is through a front vertical zipper. Two small citrus trees located in the centre of the cage may not duplicate but imitate field like situation. The cage was located in a glasshouse with temperature controls (Mutika et. al., 2001). The experiments were done once a week for four consecutive weeks.
Environmental conditions
During the experiments, temperature, humidity and atmospheric pressure were recorded every 30 minutes using a digital Meteoscope II (Augenoptikversand, Sindelfingen, Germany). Light intensity was measured from three locations within the cage; top, foliage level and floor using Lightmeter TES-1334 (TES Electrical Electronic Coorporation, Taipei, Taiwan). The observer was present in the field-cage throughout the full duration (2 and 3 hours for G. f. fuscipes and G. p. palpalis respectively) of the experiments and movements were kept minimal. The experiments were repeated four times for each of the test species. Each repeat is referred to as a run.
Mating Competitiveness
Mating propensity was defined as the proportion of females that mated, and are indicative of the tendency of the flies to mate. The relative mating index was defined as the number of mating pairs accounted for by the age category as a proportion of the total number of mating pairs. This measure represents the competitiveness of each age category tested relative to the other categories (Cayol, et. al, 1999).
Virgin male and female G. f. fuscipes and G. p. palpalis were collected from the colony on the first day of eclosion from pupae. To ensure that female and male flies were not mated, they were removed as they emerged from pupae. The experiments began at 10.00h and terminated at 13.00h local time to cover the morning peak activity hours of flies in the field. In each test species a total of 30 females were released in the field cages and observed for the presence of non-fliers. Any non-flier was removed and replaced.
Different age categories chosen following the mating regime system reported in Abila et. al. (in press). Equal numbers (n=15) of male flies, 1, 5, 8 and 13 days after eclosion, were marked with different colors on the thorax and introduced in the cage resulting in a total of 60 males in each test species. As in the case of the females, any non-flier was removed and replaced. For each mating pair that formed, the age of the male fly was identified by the age-specific color code.
Mating duration
The flies were observed carefully for any signs of male pursuit of females. Once genitalia were engaged and the pair was in copula, the pair was collected into individual tubes. Age of the male, and the starting and separation time of each successful mating, were recorded to the minute. The duration was then calculated as the difference in minutes between ending and starting times. Once copulation ended, the male was removed and the female was kept in the fly holding room overnight to allow sperm migration to the spermathecae.
Insemination
Dissections were done in physiological saline solution under binocular microscope, to determine insemination rate and spermathecal fill (Pollock, 1982). The spermathecae were removed, and mounted on a microscope slide with a cover slip. Spermathecal fill was estimated by microscopic viewing each spermatheca under 100 × power. The spermathecal fill was scored as; empty (0), quarter full (0.25), half-full (0.50), three-quarter-full (0.75) and full (1.0). The amount of sperm transferred was then computed as the mean spermathecal fill values of both spermathecae (Nash, 1955).
Data Analysis
Data were subjected to Arcsine transformation before analysis of variance and for significant variations; the homogeneity of means was tested by the Least Square Difference (LSD) procedure.
Results
Environmental conditions
Light intensity was not controlled and varied naturally. The values recorded range from 596 lux within the foliage of the citrus trees and the maximum was 11380 lux at the roof of the cage. The average light intensity was computed to have been 3944 ± 432 lux. Temperature varied from 20.0 to 28.5 °C. The average was 24.4 ± 0.4. Relative humidity ranged from 40% to 75%, with an average of 53.7 ± 1.8 %. The average atmospheric pressure during the experiments was 997 ± 1.9 mb. Figure 1 summarises the temperature and relative humidity observed.
Figure 1:
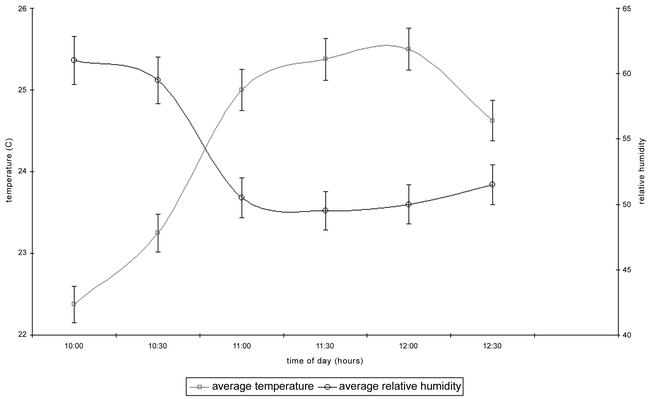
Mean temperature and humidity recorded during the experiments
Mating Competiveness
When released from the holding cages, the flies dispersed randomly throughout the field-cage. Many settled within the foliage provided by the citrus trees in the field cage. Flies preferred to rest on the lower side of the leaves and branches. Some flies rested on the roof and walls of the cage.
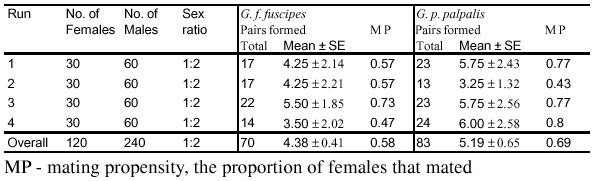
The overall mating propensity of the experiment was 0.59 ± 0.05 in G. f. fuscipes and 0.69 ± 0.18 in G. p. palpalis (see Table 1).
Table 1:
Sumary of mating results in the field cage
There was no definite pattern of pairing behavior observed in relation to time of the experiments((Figure 2). Runs 1 and 2 had similar patterns of activity except that the peak activity times varied between the 2nd and 3rd hours, respectively. The majority of pairs were formed within the first 60 minutes of the experiments. There were long periods of relative immobility followed by a sudden surge in activity and mating strikes, genital engagement and copulation.
Figure 2 a:
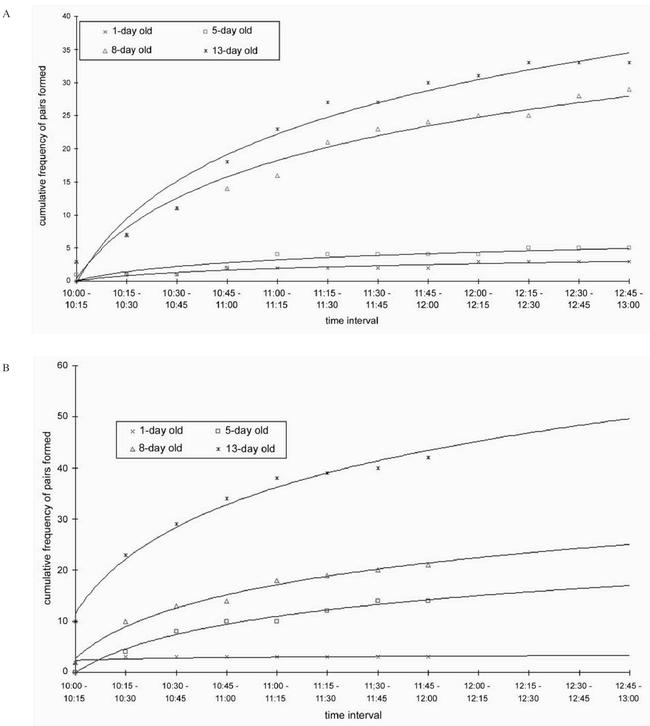
Cumulative frequency of pair formation with time in G. f. fuscipes. 2b: Cumulative frequency of pair formation with time in G. p. palpalis
During the experiments, some males were observed trying to engage genitalia with already copulating females, but were unsuccessfull in dislodging the earlier male during the occasions that this behavior was observed. In G. f. fuscipes, most (38.6%, N=70) of the mating pairs were formed within the folliage provided by the citrus plants in the cage. Another 32.9% were formed on the walls of the cage and 15.7% on the wall edges, the rest forming on the roof.
Analysis of variance revealed a highly significant difference in the relative mating indices influenced by age of male in both test species; (P≪0.001, F = 21.44, df = 3) in G. f. fuscipes and (P≪0.001, F = 65.04, df = 3) in G. p. palpalis. LSD tests reveal two groups in G. f. fuscipes in which the means were not significantly different. The 8 and 13-day old were not significantly different, but were superior to the 5 and 1-day old male. In G. p. palpalis, all the four age categories differed significantly from one another. The 13-day old males mated most frequently followed by the 8, 5, and 1-day old males in decreasing order of competitiveness. The runs did not significantly affect the variation (P∼1.0, F∼ 0, df = 3) in both species (Table 2 and Figure 3 a and b).
Table 2:
Effect of age on the competitiveness of male G. f. fuscipes and G. p. palpalis
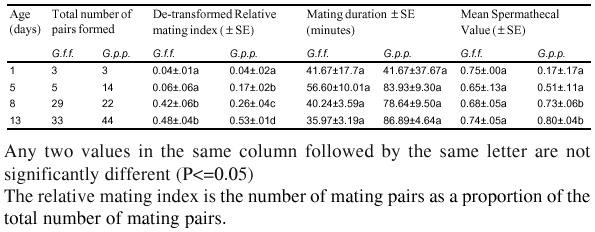
Figure 3a:
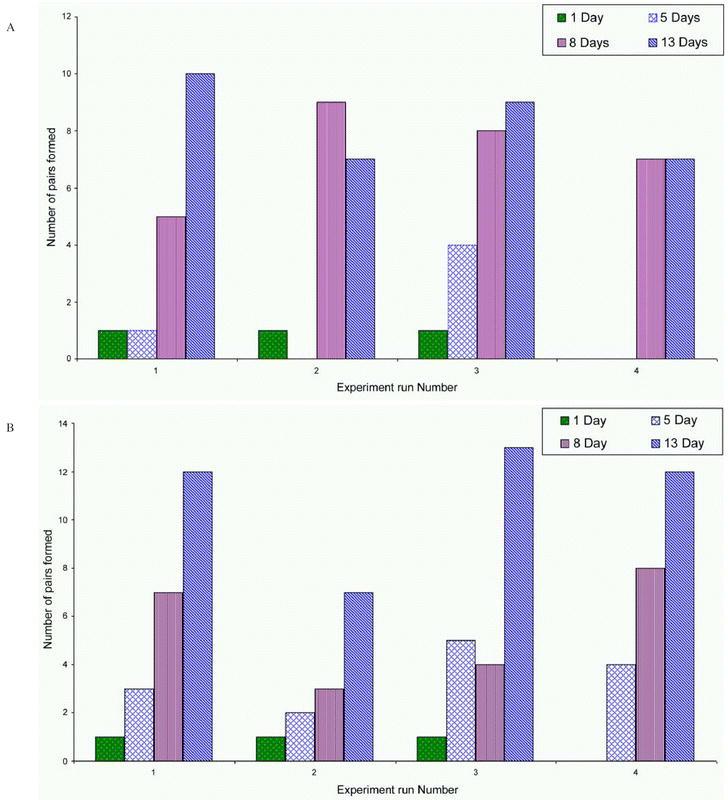
Number of pairs formed by age category against run in G. f. fuscipes. 3b: Number of pairs formed by age category against run in G. p. palpalis
Mating duration
After genital engagement copulation ensued, which had a variable duration. In both test species, no significant differences in mating duration were seen (Table 2).
Insemination
All the G. f. fuscipes females that mated (N=70) were inseminated with varying spermathecal indices. The variation in insemination indices in G. f. fuscipes were not significanly affected by the age of males (P = 0.0649, F = 2.530, df=3). Nevertheless, the mean spermathecal fill value declined with age of the male (Table 2). In G. p. palpalis age significantly affected the ability of males to inseminate (P≪0.001, F=6.74, df = 3). The 1 and 5-day old male G. p. palpalis were less able to inseminate compared to the 8 and 13 day old males. Only 6.64% (n = 8, N = 83) were not inseminated, showing an insemination rate of 93.36% among females that copulated.
Discussion
The results of this study confirm that age influences the mating success of male G. f. fuscipes and G. p. palpalis. Young males (1 and 5-day old) are less competitive in comparision to the eight and thirteen day old categories. Nevertheless, in contrast to the findings of Mellanby (1936), that G. palpalis (this has been subsequently refered to as G. f. fuscipes as reviewed by Newstead (1911)) males are unable to inseminate before 5 days, in our experiments, one day old flies (n=3) were able to score an overall mean spermithecal value of 0.75 ± 0.00. Among the tested age categories, the optimal age for release of G. f. fuscipes males was shown to be at least 8 days in this study. It is here recommended that sterile male G. f. fuscipes releases should be composed of flies that are 8 to 13-day old. In G. p. palpalis, however, the 13-day old males were shown to mate siginificantly more than the next best age (8-day old). The appropriate sterile male release age for G. p. palpalis is therefore 13-days old flies.
It would be worthwile to release the 8-day old sterile G. p. palpalis males if their longevity in the field can maintain adequate numbers upto the 13th day after emergence. A recent study addresing the effects of gamma radiation on the longevity of male G. pallidipes (Opiyo et. al. 2001) reports that longevity spaned a period of 60 days in the laboratory. Earlier studies however indicate that irradiation reduced longevity in G. p. gambiense (Politzar et. al., 1979), G. tachinoides, G. f. fuscipes, and G. brevipalpis (Vreysen et. al., 1996).
The sterile male release ages recommended here are consistent with those obtained in a similar study using fly holding cages with G. pallidipes that estimated the optimal release age at seven days (Olet et. al., 2002). However, it is possible to explain the descrepancy as a result of the systematic age categorisation (1, 5, 8, and 13 in this study while Olet et. al., 2000 used; 3, 5, 7, 9, 11, 13, and 15 day old males) and/or real differences resulting from using different species.
Regardless of the development of physiological sexual maturity, changes in the competitiveness with age may have a more fundamental influence on mating competitiveness and success across several tsetse species (Wall and Langley, 1993). In G. p. palpalis, 3 – 6 day old males were observed to account for only 18% of copulations when in competition with equal numbers of older males (Nash, 1955). Ten-day-old G. m. morsitans Westwood males inseminated significantly more females (and to a greater degree) than 5-day old males (Southon and Cockings, 1963). In a study of the effects of age on responses of G. m. morsitans and G. pallidipes males to decoy females in the laboratory, it was shown that there was a significant increase in activity levels with age, which resulted in older males mating more frequently (Wall, 1988).
The age of male flies did not significantly affect mating duration in either test species. Mating duration in both test species were in the order of 40 minutes. It has been suggested before that long copulatory periods exceeding 30 minutes are caused by unsuccessful attempts at engaging genitalia at the on-set of copulation, but no attempt was made to relate this to the age of the male fly (Mutika et. al., 2001).
This study has revealed that the age of male G. f. fuscipes and G. p. palpalis influences sexual competitiveness, but not mating duration and insemination. These results should provide a picture of actual field conditions although only laboratory maintained strains of the test species were used. These conditions approximate actual sterile insect release programs, but may fall short of the actual field scenario, where colonized and wild males compete for fertile females. Using the walk-in field cage, however, provides the nearest possible field-like conditions for this type of investigation. Any future attempts to address these concerns in a single experiment will go a long way in improving our understanding of the reproductive behavior and factors affecting the competitiveness of released sterile males.
Acknowledgments
This study was part of IAEA fellowship awards to Patrick Abila P'Odyek and Martin Kiendrebeugo for training in the field of Entomology. Special appreciation is extended to Rudolf Boigner and Geaty Germershausen who looked after the experimental flies; Henry Adun, Cuthbert Daffa, and Christopher Lukiko for their advice in fly handling through the experiments. We also thank the two anonymous reviewers for their useful comments on the original manuscript.
References
- Abila PP, Okedi LM, Luyimbazi F, Ogwal LM, and Olaho-Mukani W. Rearing of Glossina fuscipes fuscipes at Tororo, for the Proposed SIT project for Buvuma Islands on Lake Victoria, Uganda. Proceedings of the 26th meeting of the International Scientific Council for Trypanosomiasis Research and Control. (ISCTRC), 1–5 October 2001: Ouagadougou, Burkina Faso. (in-press). [Google Scholar]
- Alcock J. Post-insemination associations between males and females in insects: The mate-guarding hypothesis. Annual Review of Entomology. 1994;39:1–21. [Google Scholar]
- Allsopp R. Options for vector control against Trypanosomiasis in Africa. Trends in Parasitology. 2001;17:15–19. doi: 10.1016/s1471-4922(00)01828-6. [DOI] [PubMed] [Google Scholar]
- Alphey L, Andreason M. Dominant lethality and insect population control. Molecular and Biochemical Parasitology. 2002;121:173–178. doi: 10.1016/s0166-6851(02)00040-3. [DOI] [PubMed] [Google Scholar]
- Brightwell RR, Dransfield D, Stevenson P, Williams B. Changes over twelve years in populations of Glossina pallidipes and Glossina longipennis (Diptera: Glossinidae) subject to varying trapping pressure at Nguruman, southwest Kenya. Bulletin of Entomological Research. 1997;87:349–370. [Google Scholar]
- Budd L. 1999 DFID-Funded tsetse and Trypanosomiasis research and development since 1980: Vol. 2. An Economic Analysis, Department of International Development, Livestock Production Programme, NRIL, Chatham, Kent, UK. [Google Scholar]
- Calkins CO, Webb JC. A cage and support framework for behavioral tests of fruit flies in the field. Florida Entomologist. 1983;66:512–514. [Google Scholar]
- Carlson DA, Schlein Y. Unusual polymethyl alkanes in tsetse flies acting as abstion in Glossina morsitans. Journal of Chemical Ecology. 1991;17:267–284. doi: 10.1007/BF00994332. [DOI] [PubMed] [Google Scholar]
- Cayol JP, Vilardi J, Rial E, Vera MT. New indices and methods to measure the sexual compatibility and mating performance of Ceratitis capitata (Diptera: Tephritidae) laboratory reared strains under field cage conditions. Journal of Economic Entomology. 1999;92:140–145. [Google Scholar]
- Curtis CF, Andreason MH. 1999 Large-scale control of mosquito vectors of disease. In: Tan, K. H. editor. Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, Malaysia. 135–142. [Google Scholar]
- Dyck VA, Pan H, Kassim SS, Suleiman FW, Mussa WA, Saleh KM, Juma KG, Mkonyi PA, Holland WG, van der Eerden BJM, Dwinger RH. Monitoring the incidence of trypanosomiasis in cattle during the release of sterilised tsetse flies on Ungunja Island, Zanzibar. Revue d'Elevage et de Medicine Veterinaire des Pays Tropicaux. 2000;53:239–243. [Google Scholar]
- Feldmann U. 1994a Guidelines for the rearing of tsetse flies using the membrane feeding technique. In: Ochieng-Odero, J. P. R. editor. Techniques of the Rearing for Development of Integrated Pest and Vector Management Strategies, ICIPE Science Press, Nairobi. 449–471. [Google Scholar]
- Feldmann U. 1994b Some quality control parameters used in the rearing of tsetse flies. In: Ochieng-Odero, J. P. R. editor. Techniques of the Rearing for Development of Integrated Pest and Vector Management Strategies, ICIPE Science Press, Nairobi. 113–30. [Google Scholar]
- Feldmann U, Hendrichs JH. 2001 Integrating the sterile insect technique as a component of Area-wide tsetse and Trypanosomosis Intervention. Food and Agriculture Organisation of the United Nations, Rome, PAAT Technical and Scientific Series 3. [Google Scholar]
- Forster WA. Male sexual maturation of tsetse flies Glossina morsitans Westwood and G. austeni Newstead (Diptera; Glossinidae) in relation to blood feeding. Bulletin of Entomological Research. 1976;66:389–400. [Google Scholar]
- Hendrichs J, Franz G, Rendon P. Increased effectiveness and applicability of the sterile insect technique through male-only release for control of Mediterranean fruit-flies during fruiting seasons. Journal of Applied Entomology. 1995;119:371–377. [Google Scholar]
- Knipling EF. Possibilities of insect population control through the use of sexually sterile males. Journal of Economic Entomology. 1955;48:459–462. [Google Scholar]
- Knipling EF. The sterile male method of population control. Science. 1959;130:902–904. doi: 10.1126/science.130.3380.902. [DOI] [PubMed] [Google Scholar]
- Knipling EF. 1963 Potential role of the sterility principle for tsetse fly eradication. WHO/Vector control/27, WHO/EBL/9:77. [Google Scholar]
- Leak SGA. 1999 Tsetse Biology and Ecology: their role in the epidemiology and control of Trypanosomiasis. CABI Publishing, New York. [Google Scholar]
- Lindquist DA, Abusowa M, Hall MJ. The New World screw worm fly in Libya: A review of its introduction and Eradication. Journal of Medical and Veterinary Entomology. 1992;6:2–8. doi: 10.1111/j.1365-2915.1992.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Mellanby K. Experimental work with the tsetse fly, Glossina palpalis in Uganda. Bulletin of Entomological Research. 1936;27:611–632. [Google Scholar]
- Mutika GN, Opiyo E, Robinson AS. Assessing mating performance of male Glossina pallidipes Austen (Diptera: Glossinidae) using a walk-in field cage. Bulletin of Entomological Research. 2001;91:281–287. doi: 10.1079/ber2001102. [DOI] [PubMed] [Google Scholar]
- Nash TAM. The Fertilisation of Glossina palpalis in captivity. Bulletin of Entomological Research. 1955;46:357–368. [Google Scholar]
- Newstead R. A revision of the tsetse-flies (Glossina) based on a study of the male genital armature. Bulletin of Entomological Research. 1911;2:9–36. [Google Scholar]
- Olandunmade MA, Feldmann U, Takken W, Tenabe SO, Hamann HJ, Onah J, Dengwat L, Van Der Vloedt AMV, and Gingrich RE. 1990 Eradication of Glossina palpalis palpalis (Robineau Devoidsady) (Diptera: Glossinidae) from agropastoral land in central Nigeria by means of the sterile insect technique. In: Sterile Insect Technique for Tsetse Control and Eradication. International Atomic Energy Agency, Vienna. [Google Scholar]
- Olet PA, Opiyo E, Robinson AS. Sexual Receptivity and age in Glossina pallidipes Austen (Dipt., Glossinidae) Journal of Applied Entomology. 2002;126:86–91. [Google Scholar]
- Opiyo E. Survival and reproductive potential of gamma irradiated male Glossina pallidipes Austen. Entomologia Experimentalis et Applicata. 2001;99:397–400. [Google Scholar]
- Patterson RS, Lowe RE, Smittle BJ, Dame DA, Boston MD, Cameron AL. Release of Radiosterilised males to Control Culex pipiens quinquefasciatus (Diptera: Culicidae) Journal of Medical Entomology. 1977;14:299–304. [Google Scholar]
- Politzar H, Merot P, Brandl FE. Experimental aerial releases of sterile males of Glossina palpalis gambiensis and Glossina tachinoides in a biological control operation. Revue d'Elevage et de Medicine Veterinaire des Pays Tropicaux. 1984;37:198–202. [PubMed] [Google Scholar]
- Politzer H, Cuisance D, Lafaye A, Clair M, Taze Y, Sellin E. Experimentation sur le terrain de la lutte genetique par lachers de males steriles: longevite et dispersion des males de Glossina palpalis gambiensis (Haute Volta) Annals Sociologique la Belgique pour la Medicine Tropicale. 1979;59:59–78. [PubMed] [Google Scholar]
- Pollock JN. 1982 Training Manual for Tsetse Control Personnel. Vol. 1: Tsetse biology, systematics and distribution, techniques. Food and agricultural Organization of the United Nations, Rome. [Google Scholar]
- Southon HAW, Cockings KL. 1963 Laboratory maintenance of Glossina. Annual Report of the East African Trypanosomiasis Research Organisation (1961). [Google Scholar]
- Thomson JW, Wilson A. 1991 The control of tsetse flies and trypanosomiasis by the application of deltamethrin to cattle. Proceedings of the 20th Meeting of the International Scientific Council for Trypanosomiasis Research and Control (ISCTRC), Mombassa, Kenya, April 1989. 450–454. [Google Scholar]
- Van der Vloedt AMV, Taher M, Tenabe SO. Effect of gamma radiation on tsetse fly Glossina palpalis palpalis (Rob.-Desv.) (Diptera: Glossinidae) with observations on reproductive biology. International Journal of Applied Radiation and Isotopes. 1978;29:713–716. doi: 10.1016/0020-708x(78)90117-5. [DOI] [PubMed] [Google Scholar]
- Vreysen M.B.J, Van der Vloedt A.M.V., Barnor H. Comparative gamma radiation sensitivity of G. tachinoides Westw., G. f. fuscipes Newst., and G. brevipalpis Newst. International Journal of Radiation Biology. 1996;69:67–74. doi: 10.1080/095530096146192. [DOI] [PubMed] [Google Scholar]
- Vreysen MJB, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, Dyck AV, Msangi AR, Mkonyi PA, Feldmann U. Glossina austeni (Diptera: Glossinidae) Eradicated on the Island of Ungunja, Zanzibar using the sterile Insect Technique. Journal of Economic Entomology. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- Wall R. Tsetse mating behavior: effects of age and hunger in Glossina m. morsitans and G. pallidipes. Physiological Entomology. 1988;13:479–486. [Google Scholar]
- Wall R, Langley PA. The mating behavior of tsetse flies (Glossina): a review. Physiological Entomology. 1993;18:211–218. [Google Scholar]
- Williamson DL, Dame DA, Gates DB, Cobb PE, Bakuli B, Werner PV. Integration of insect sterility and insecticides for control of Glossina morsitans morsitans Westwood (Diptera: Glossinidae) in Tanzania: The impact of sequential releases of sterilised flies. Bulletin of Entomological Research. 1983;73:393–404. [Google Scholar]


