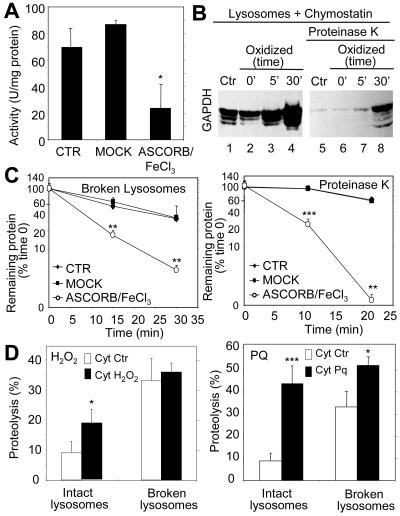
Figure 2.
Oxidation of CMA substrates facilitates their uptake by lysosomes. (A) GAPDH was exposed to an ascorbic acid/iron oxidizing reaction for 30 min as described under Materials and Methods. The remaining specific enzymatic activity of untreated GAPDH (CTR) and of GAPDH exposed to the oxidant mixture (ASCORB/FeCl3) or to the dialysis buffer only (MOCK) is shown. Values are mean ± SE of two different preparations with triplicate measurements. (B) Unmodified GAPDH (Ctr) or GAPDH exposed to the oxidant mixture for the indicated periods of time was incubated for 30 min under standard conditions with intact chymostatin-treated liver lysosomes isolated from 20-h starved rats. Where indicated, samples were treated with proteinase K to remove GAPDH bound to the cytosolic side of the lysosomal membrane. At the end of the incubation, lysosomes were collected by centrifugation and subjected to SDS-PAGE and immunoblot for GAPDH. (C) The different forms of GAPDH (50 μg) described in A were incubated with lysosomal enzymes (25 μg of protein of broken lysosomes; pH 4.5) (left) or proteinase K (0.1 μg) (right) at 37 or 0°C, respectively. At the indicated times aliquots were taken and subjected to SDS-PAGE and Coomassie Blue staining. Values are the mean + SE of the densitometric values for GAPDH from three different experiments. (D) Mouse fibroblasts were metabolically labeled with [3H]leucine for 48 h. A group of fibroblasts was exposed to 100 μM H2O2 (left) or 40 μM paraquat (Pq) for 1 h during the labeling. After cavitation, cytosolic fractions (Cyt) from untreated (Ctr) and H2O2 or paraquat-treated fibroblasts were prepared and incubated for 30 min at 37°C with intact rat liver lysosomes (25 μg of protein) or with lysosomes disrupted by a hypotonic shock (broken lysosomes; 15 μg of protein) under standard conditions. At the end of the incubation proteolysis was calculated as the amount of acid precipitable radioactivity (protein) transformed in acid soluble (amino acids and small peptides). Values are the mean + SE of six different experiments (*p < 0.05, **p < 0.01, ***p < 0.001).