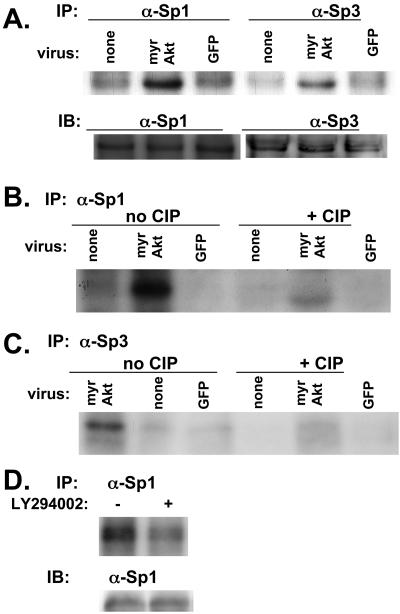
Figure 7.
myrAkt is associated with phosphorylation of Sp1 and Sp3 in SF188 cells. SF188 cells were infected with adenovirus expressing myrAkt or GFP. Thirty-six hours after infection, cells were in vivo–labeled with orthophosphate. In the top part of A labeled IP, in vivo–labeled proteins were immunoprecipitated with either Sp1 or Sp3 antibody as indicated. Immunoprecipitated complexes were separated on 10% SDS-PAGE gel and transferred to nitrocellulose membrane and autoradiographed. In the bottom part of A labeled IB (immunoblot), these same lysates were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membrane, and probed with either Sp1 or Sp3 antibody to serve as a loading control. (B) The same procedure used in A was followed, but after immunoprecipitation with anti-Sp1 antibody, each protein extract was incubated with or without 10 mU of calf intestinal phosphatase (CIP) for 30 min at room temperature before running on an SDS-PAGE gel. The identical procedure was followed for C as in B, except lysates were immunoprecipitated with an anti-Sp3 antibody. (D) U87MG cells were treated with LY294002 (20 μM) or DMSO (control) for 16 h. At this time, cells were lysed and the procedure described for A was followed.