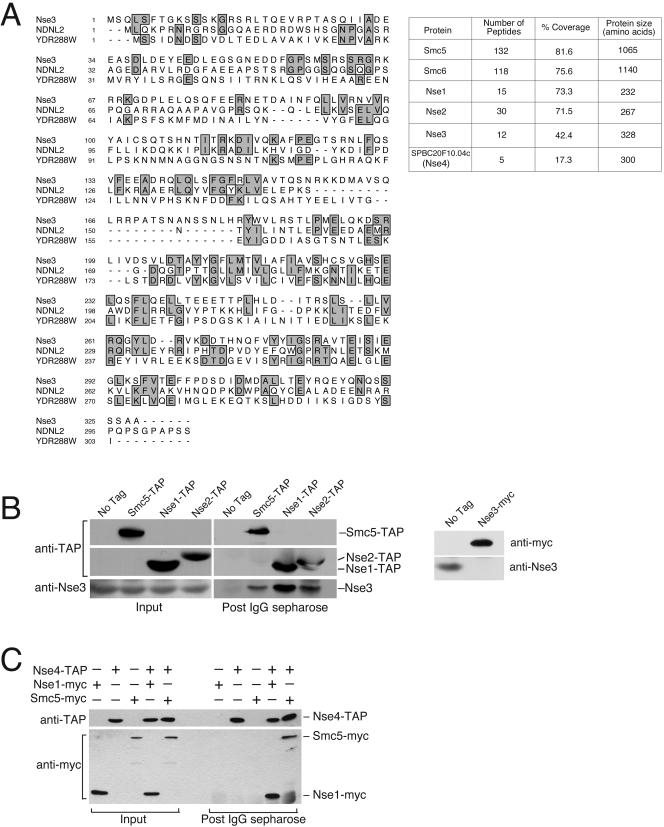
Figure 1.
Identification of Nse3, a novel Smc5+6 complex subunit. (A) Shows an alignment between fission yeast Nse3 (SPCC645.04), human NDNL2 (NP_619649.1) and budding yeast YDR288W. Regions of identity are boxed and shaded. The table on the right shows the number of Smc5, Smc6, Nse1, Nse2, Nse3, and SPBC20F10.04c (Nse4) peptides obtained in mass spectrometric analysis of Smc5-associated proteins. The primary sequence coverage and size of Smc5, Smc6, Nse1, Nse2, Nse3, and SPBC20F10.04c (Nse4) are also given. (B) Confirmation that Nse3 is part of the Smc5+6 complex. Smc5-TAP, Nse1-TAP, and Nse2-TAP proteins, expressed from their endogenous loci, were purified on IgG-Sepharose and the copurifying proteins were analyzed by Western blotting with a polyclonal anti-Nse3 antibody. The right-hand panel shows the specificity of the anti-Nse3 antibody. Nse3 is essential so to test the specificity of anti-Nse3 sera we probed total extracts from cells expressing endogenous Nse3 tagged, or not, with a 13myc epitope. A band of ∼35 kDa is detected with anti-Nse3 in cells without an epitope tag on Nse3. This band is absent in cells in which Nse3 is tagged with a 13myc epitope and is replaced by a lower mobility band corresponding to Nse3-myc that is detected by both anti-Nse3 and anti-myc antisera (anti-myc shown).