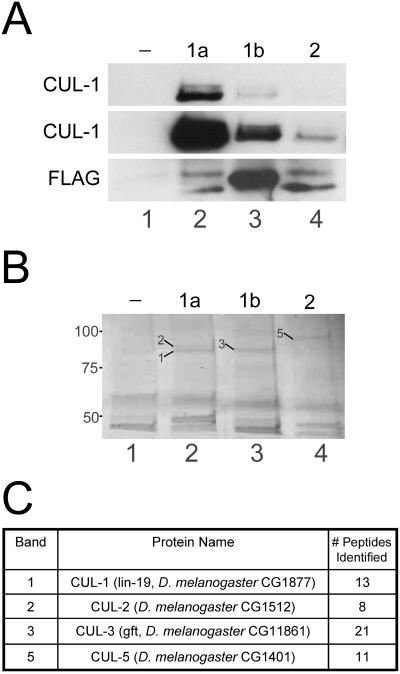
Figure 6.
The three Drosophila Roc proteins preferentially bind different Cullins. (A) Lysates from embryos expressing no transgene (-; lane 1) or one of the Roc1agrf::FLAG-Roc proteins (1a, 1b, or 2; lanes 2-4) were incubated with anti-FLAG agarose and immunocomplexes were analyzed by Western blot with anti-CUL-1. Top, short exposure showing that Roc1a efficiently immunoprecipitates both neddylated and unmodified CUL-1. Middle, longer exposure of the same blot showing that Roc1b and Roc2 can also immunopurify CUL-1, although much less efficiently than Roc1a. Bottom, blot probed with anti-FLAG to demonstrate immunoprecipitation of the FLAG-Roc proteins. The slower migrating bands in lanes 2 and 4 correspond to unidentified proteins, possibly read-through translation products. (B and C) Immunocomplexes from the embryo lysates of the same genotypes as in A were analyzed by mass spectrometry. (B) Coomassie-stained gel showing that each Roc protein immunoprecipitates a unique set of proteins in the size range expected for the Cullins. Bands indicated with arrows were excised and analyzed by mass spectrometry (the corresponding Cullin protein is identified by the number). (C) Protein identification of the bands excised from the gel in B. CUL-1 and CUL-2 were each identified FLAG-Roc1a immunocomplexes, CUL-3 was identified in FLAG-Roc1b immunocomplexes, and CUL-5 was identified in FLAG-Roc2 immunocomplexes. The number of peptides matching a theoretical digest of the corresponding protein is indicated.