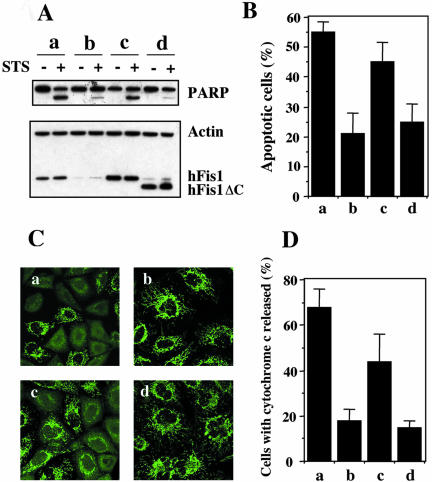
Figure 5.
Overexpression of hFis1 wild-type (hFis1 wt) but not C terminus-truncated mutant (hFis1 ΔC) reverts apoptosis-resistant hFis1 RNAi cells. HeLa cells were transfected with control shRNA/pREP4 (a), hFis1 shRNA/pREP4 (b), hFis1 shRNA/pREP4 plus hFis1wt cDNA/pcDNA3.1 (c), or hFis1 shRNA/pREP4 plus hFis1ΔC cDNA/pcDNA3.1 (d), and transfectants were selected as described in Materials and Methods. (A) Transfectants of each group (a–d) were treated with or without STS (1 μM) for 6 h, lysed, and analyzed for hFis1 expression levels and PARP cleavage by Western blotting. Actin also was analyzed as a loading control. (B) Transfectants of each group (a–d) were treated with STS (1 μM; 6 h), and the nuclei were stained with Hoechst 33342 (1 μg/ml; 15 min at RT). Normal or apoptotic nuclei of these cells in several fields were counted under the fluorescent microscope (for UV excitation). At least 200 cells altogether in each treatment were counted and shown as a percentage of cells with apoptotic nuclei among the total cells counted. The data are plotted as the mean ± SD of at least three independent experiments. (C) Transfectants of each group (a–d) were treated with Act D (10 μM; 8 h) in the presence of zVAD-fmk (50 μM), fixed, and stained with anti-cytochrome c (mouse monoclonal, green) antibodies, and images were captured by confocal microscopy. (D) The number of cells whose cytochrome c was released was counted and plotted as a percentage of the total cells counted in each group (a–d) of transfectants that had been treated with Act D and stained with anti-cytochrome c (the same samples as shown in C). At least 200 cells were counted altogether in several fields. Data are plotted as the mean ± SD of at least three independent experiments.