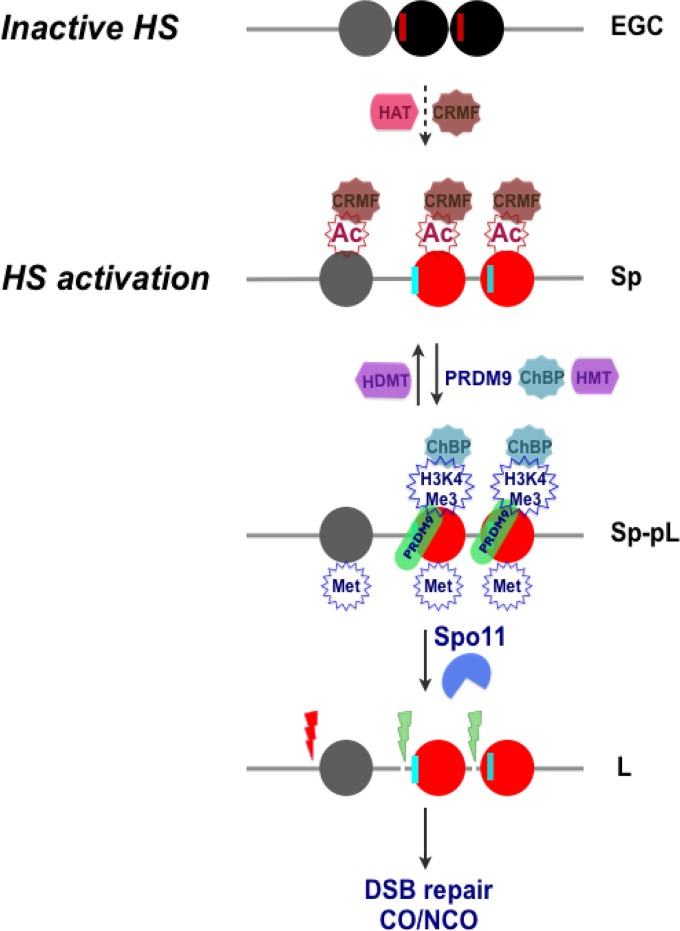
FIG 8.
Model for meiotic recombination hot spot (HS) activation by histone acetylation. (Top) A condensed chromatin structure is proposed to be manifest at the HS22 hot spot core in early germ cells (EGC), as represented by three central, closely juxtaposed nucleosomes at the core (black and gray circles). Potential PRDM9 binding sites (red vertical lines) are positioned next to “black” nucleosomes (Fig. S12A and Data Set S3). (Second panel) Licensing of hot spot cores for activation is posited to occur in spermatogonia (Sp) and preleptotene (pL) meiotic cells and requires deposition of acetylated marks on histones H3 and H4 that are directed by HATs expressed in these cells (Fig. 1A; Fig. S12B). This leads to the formation of open chromatin, which is likely facilitated by binding of chromatin remodeling factors (CRMFs) to acetylated histone tails (18, 38, 99). (Third panel) Acetylated open chromatin (red nucleosomes) then allows PRDM9 binding at its cognate binding sequences (vertical cyan lines), followed by its deposition of H3K4Me3 marks, as well as the deposition of other active methylated histone H3 marks, by HMTs expressed in these cells (Fig. 1B; Fig. S12B). (Bottom) DNA regions juxtaposed to active (red) nucleosome sites then become accessible for DSB cleavage by Spo11 at the leptotene (L) stage, which appears to occur via the recruitment of chromatin binding proteins (ChBP) to H3K4Me3 tails (11, 38, 100). DSBs are then repaired by the meiotic recombination machinery, leading to a CO or to gene conversion without a CO (NCO).