FIGURE 1.
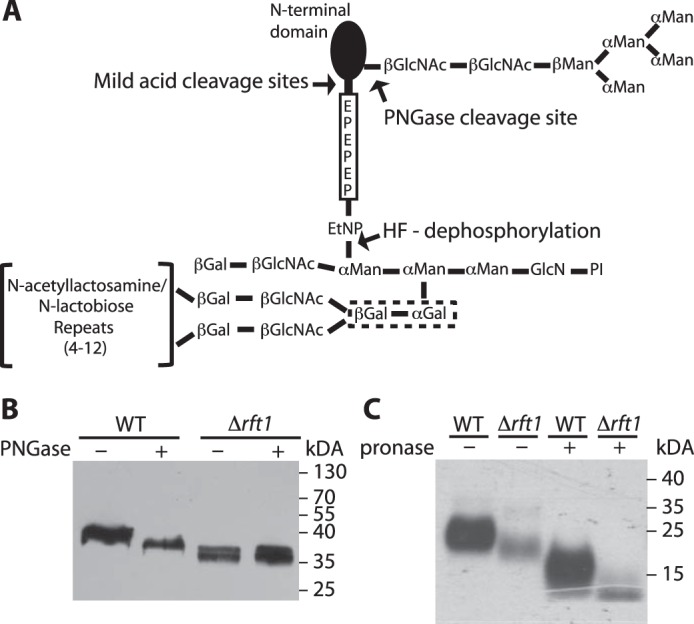
Schematic representation of procyclin glycosylation and analysis of GPI-anchored proteins in TbRFT1 null cells. A, most procyclin isoforms (except EP2 and GPEET) are modified by a homogeneous triantennary Man5GlcNAc2 glycan near the N terminus. The GPI anchor of all procyclins is modified with several N-acetyllactosamine or lacto-N-biose repeats, which may be capped with sialic acids depending on the presence of blood sialoglycoconjugates. These repeats are linked to the middle mannose of the GPI anchor core via two consecutive galactose residues (dotted box), which are probably added in the ER as suggested in Fig. 2. The C-terminal regions of EP procyclins consist of 22–30 Glu-Pro repeats. PI, phosphatidylinositol. B, immunoblotting analysis of EP procyclins isolated from WT and TbRFT1 null (Δrft1) cells. Denatured proteins were treated with (+) or without (−) PNGase F to remove N-glycans and then separated by SDS-PAGE. After electrotransfer to membranes, EP was visualized by enhanced chemiluminescence using anti-EP antibody and HRP-conjugated anti-mouse IgG. C, [3H]ethanolamine labeling and fluorography of GPI-anchored proteins from WT and TbRFT1 null (Δrft1) cells. Trypanosomes were grown in the presence of [3H]ethanolamine, and GPI-anchored proteins were extracted from the delipidated protein pellet using 9% butan-1-ol. Extracts incubated in the absence (−) or presence (+) of Pronase to remove the protein portions of GPEET and EP were separated by SDS-PAGE and analyzed by fluorography.
