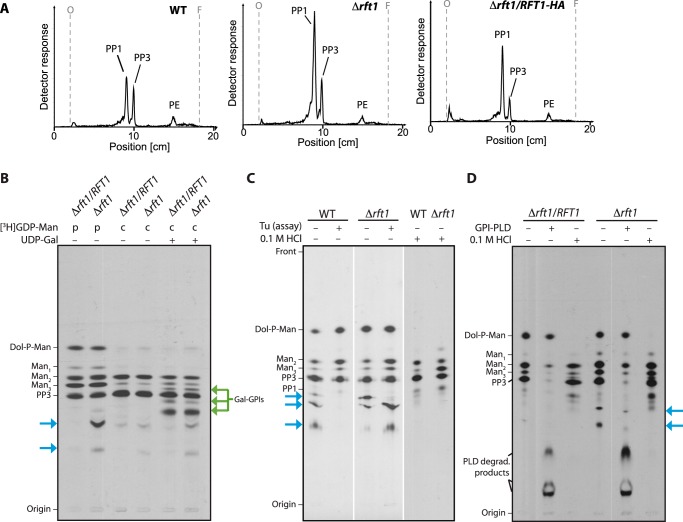
FIGURE 4.
Analysis of GPI precursor formation. A, analysis of in vivo [3H]Etn-labeled GPI precursors PP3 and PP1 extracted from WT, TbRFT1 knock-out (Δrft1), and addback (Δrft1/RFT1) cells. Trypanosome densities were adjusted before the addition of [3H]Etn to the cultures. After 4 h of labeling, GPI precursors were extracted, separated by TLC, and visualized using a radioactivity TLC scanner. The migration of PP1, PP3 (50), and phosphatidylethanolamine (PE) is indicated. B–D, in vitro [3H]GDP-mannose (GDP-Man) labeling of GPI precursors. B, membranes from hypotonically lysed TbRFT1 knock-out (Δrft1) and addback (Δrft1/RFT1) cells were pulse-labeled (p) with [3H]GDP-Man, followed by a chase (c) with non-radioactive GDP-Man in the presence (+) or absence of (−) UDP-galactose. 3H-labeled glycolipids were extracted, separated by TLC, and visualized by fluorography. Galactosylated GPI intermediates are indicated with green arrows. Blue arrows indicate the additional [3H]GDP-mannose-containing species formed in Δrft1 extracts. C, analysis of 3H-labeled glycolipids from wild-type and Δrft1 after a 30-min labeling with [3H]GDP-Man in the presence (+) or absence (−) of tunicamycin (Tu) (lanes 1–4). Lanes 5 and 6 show aliquots labeled in absence of tunicamycin that were treated with 0.1 m HCl before TLC. D, biochemical analysis of 3H-labeled glycolipids from Δrft1 and Δrft1/RFT1 after a 30-min labeling with [3H]GDP-Man in the presence (+) of tunicamycin. Primary lipid extracts were split and treated with GPI-specific phospholipase D (GPI-PLD) or 0.1 m HCl as indicated. Lipids were re-extracted after treatment and separated by TLC along with an aliquot of untreated primary extract.