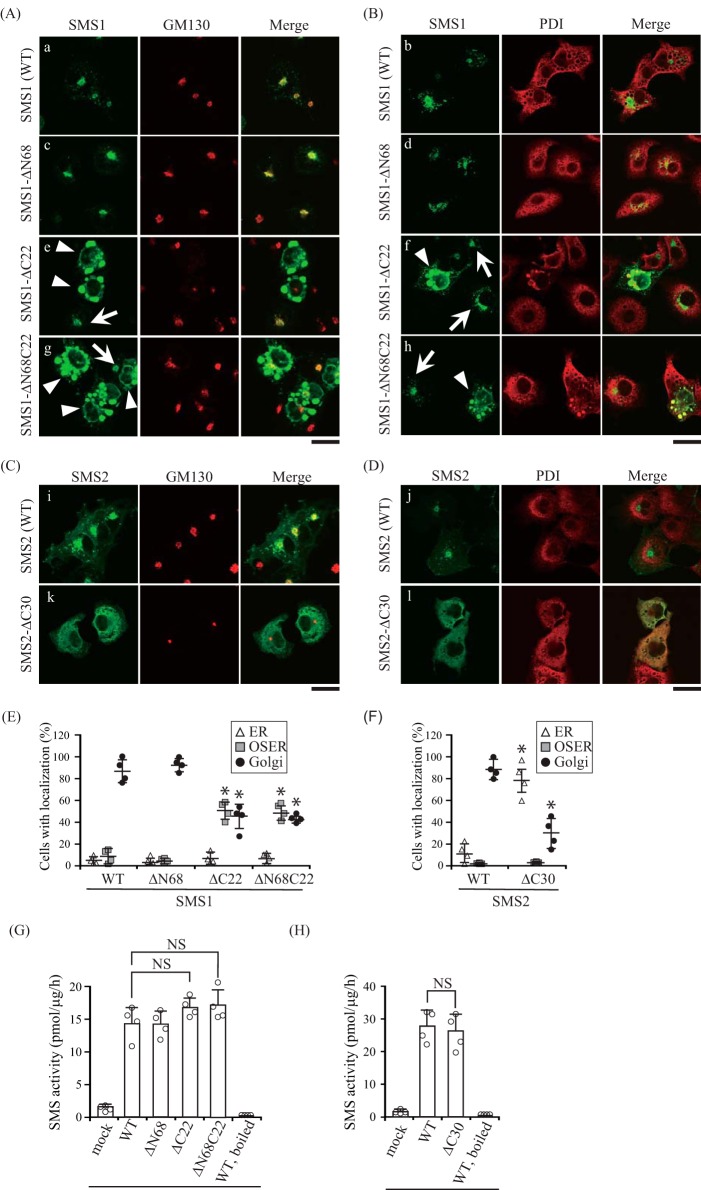
FIGURE 9.
C-terminal truncation mutants decrease the Golgi localization of SMSs. A–F, COS7 cells were transfected with a plasmid encoding C-terminal V5-tagged SMS1-WT, SMS1-ΔN68, SMS1-ΔC22, or SMS1-ΔN68C22 (A, B, and E), or SMS2-WT or SMS2-ΔC30 (C, D, and F). 24 h post-transfection, the cells were stained with anti-V5 antibody (SMS1 or SMS2), followed by appropriate Alexa Fluor-conjugated secondary antibodies, and analyzed by confocal microscopy. Localization was confirmed by co-staining with antibodies against the Golgi marker GM130 (A and C) or the ER marker PDI (B and D). SMS1 or SMS2, green; GM130 or PDI, red. Scale bars = 40 μm. Arrows and arrowheads indicate the localization of SMS1 at the Golgi apparatus and OSER, respectively. E and F, SMS localization was assessed in at least 150 cells using confocal microscopy, and the localization ratio was calculated by dividing the number of cells showing subcellular protein localization by the total number of observed cells. Individual data points are shown as a scatterplot. Values represent the mean ± S.D. from four independent experiments. *, p < 0.01. G and H, SMS activity in the lysates of COS7 cells expressing WT or truncated mutants were determined using C6-NBD-Cer as a substrate. Reaction mixtures containing cell lysates (10 μg of protein/assay) were incubated at 37 °C for 30 min. Individual data points are shown as a scatterplot. Values represent the mean ± S.D. from four independent experiments. *, p < 0.01; NS, not significant.