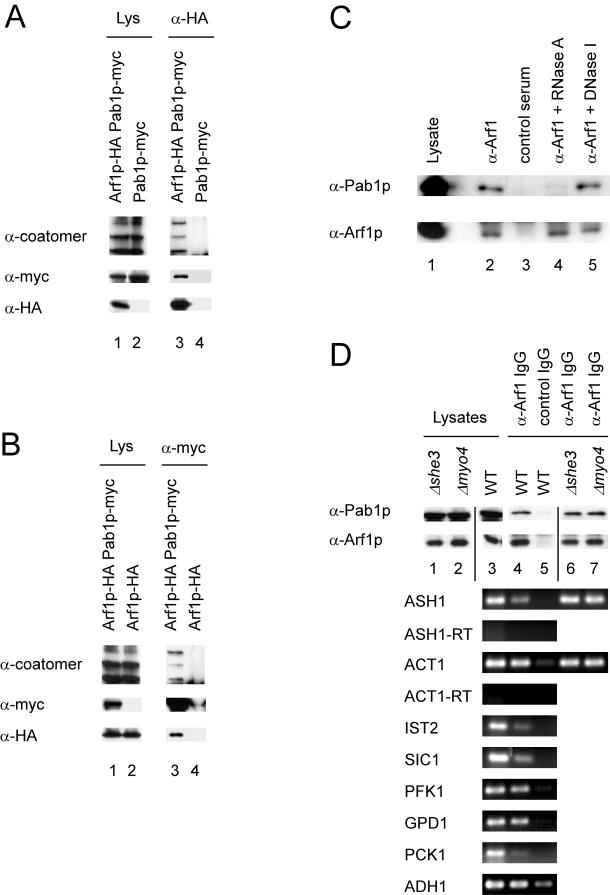
Figure 3.
Arf1p and Pab1p are present in a ribonucleotide particle. (A and B) Pab1p and Arf1p coimmunoprecipitate. Pab1p and Arf1p were chromosomally appended with either a myc- or HA-tag. Yeast lysates were prepared from single- or double-tagged strains and subjected to immunoprecipitation with anti-myc or anti-HA antibodies. The precipitates were analyzed by immunoblot. Lanes 1 and 2 correspond to 1.7% of the lysate. (C) Pab1p–Arf1p interaction depends on mRNA. Yeast lysate from a wild-type strain was incubated with RNase A, DNase I, or mock treated. After the treatment an immunoprecipitation was performed with anti-Arf1p serum or a control serum and protein A-Sepharose. The precipitated proteins were detected by immunoblot. In lane 1, 1.7% of the lysate was loaded. (D) ASH1 mRNA is part of the Pab1p-Arf1p ribonucleotide particle even in the absence of the SHE machinery. A coimmunoprecipitation experiment was performed with affinity-purified anti-Arf1p antibodies. RNA was prepared from the precipitate and subjected to RT-PCR with primer specific for the indicated mRNAs. -RT indicates reactions in the absence of reverse transcriptase. In lanes 1 and 2, 1.7% of the lysate was loaded.