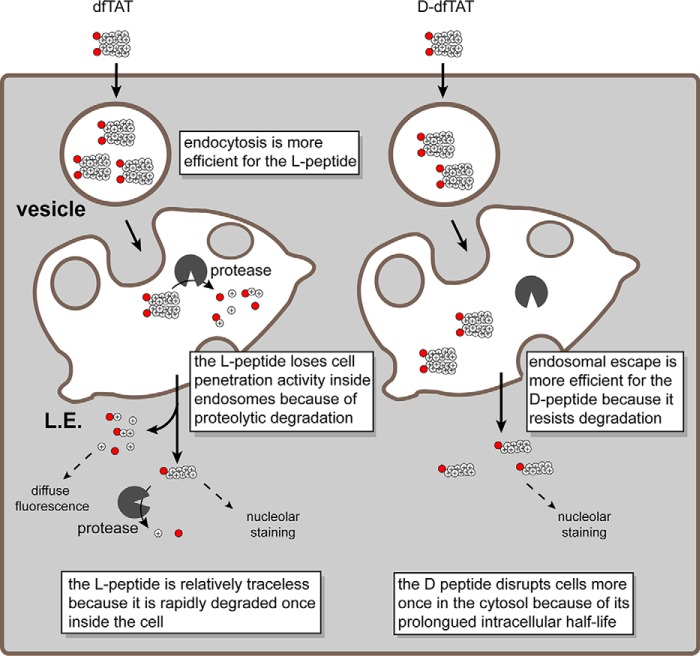
FIGURE 6.
Schematic representation modeling the similarities and differences observed in the cellular penetration of dfTAT and d-dfTAT. dfTAT is internalized by endocytosis at a higher level than d-dfTAT. Along the endocytic pathway, dfTAT is partially degraded by proteolytic enzymes, whereas d-dfTAT is not. Upon reaching the late endosome, both dfTAT and d-dfTAT induce the leakage of BMP-containing membranes. Due to proteolytic degradation, the endosomal escape efficiency of dfTAT is, however, diminished compared with d-dfTAT. Upon escaping from late endosomes, d-dfTAT and intact dfTAT are represented as being reduced to their monomer counterparts in the cytosol (e.g. by the action of glutathione). The peptide then diffuses into the cytosolic space and accumulates at nucleoli. The degradation products of dfTAT contribute to a diffuse cytosolic fluorescent distribution. d-dfTAT remains intact inside cells for several days and impacts the physiology of cells negatively. In contrast, cytosolic enzymes degrade dfTAT in a matter of hours. This in turn leads to a dramatically diminished physiological impact.