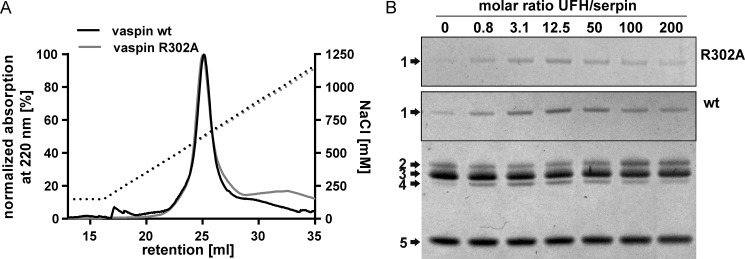
FIGURE 3.
Heparin binding and activation of vaspin R302A. A, heparin affinity chromatography of vaspin WT and mutant R302A. Elution via a NaCl gradient (black dotted line) was monitored at 220 nm. B, shown are Coomassie-stained SDS gels of a fixed molar ratio (1:3) of serpin and KLK7 incubated in the presence of increasing concentrations of UFH (0–200-fold inhibitor) for 1 min (vaspin WT, bottom gel) or 30 min (vaspin R302A, top gel). For the R302A mutant, only the complex bands are shown. Although R302A is a dramatically slower inhibitor, complex formation is accelerated, comparable with vaspin WT.