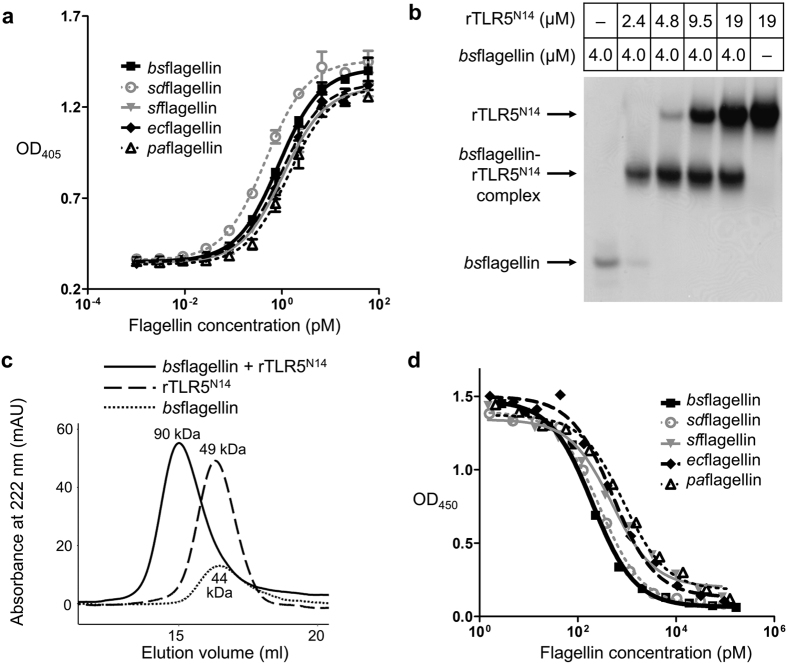
Figure 1. TLR5 interaction and activation by flagellins.
(a) TLR5 signaling activities of bsflagellin, sdflagellin, paflagellin, sfflagellin, and ecflagellin. Activities were determined in duplicate using the HEK293TLR5 reporter cell assay. The data [means ± standard deviation (S.D.); n = 2] are representative of three independent experiments that yielded similar results (Table 1). (b) Native PAGE analysis of the direct interaction between bsflagellin and rTLR5N14. (c) Gel-filtration analysis of the complex formation between bsflagellin and rTLR5N14. The apparent molecular weight of each peak was estimated using the elution volumes of gel-filtration standards and is shown near the peak. Protein elution was monitored by optical absorbance at 222 nm instead of 280 nm because bsflagellin does not contain any tryptophan and tyrosine residues that are detectable with 280 nm absorbance. (d) rTLR5N14-binding capacity of bsflagellin, sdflagellin, paflagellin, sfflagellin, and ecflagellin determined with the competitive binding assay. The data shown are representative of at least three independent experiments that yielded similar results (Table 1).