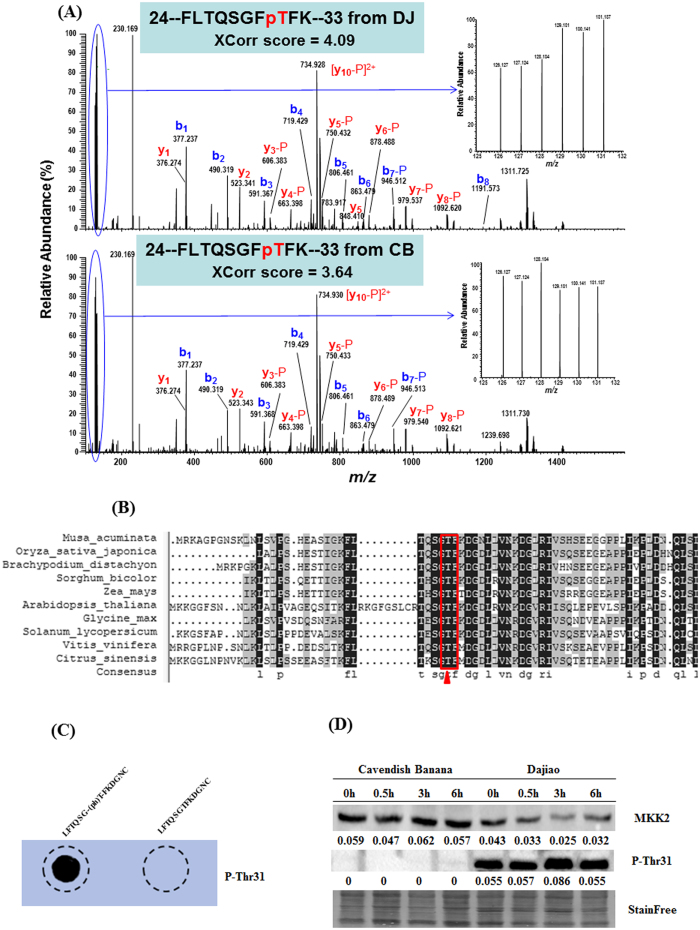
Figure 5. Identification and validation of a T31 phopshopeptide of MKK2 protein in response to cold stress.
(A) MS/MS Spectra of a doubly-charged ion at m/z 783.9192+ confidently identifying a TMT-labeled tryptic peptide with T31 phosphorylation from MKK2 protein (GSMUA_Achr7P09660_001) in both Dajiao (top panel with Xcorr score at 4.09) and Cavendish Banana (bottom panel with Xcorr score at 3.64). The inset of each panel shows the expanded view of relative intensity of 6 TMT reporter ions for determining the abundance changes of the T31 phosphopeptide in response to cold stress at 0 h in triplicate (126, 127, 128) versus 3 h (129, 130, 131). (B) Amino acid sequence alignment of MKK2 between Musa spp. and nine species indicates that T31 residue is conserved among all important plant species, suggesting the phosphorylation of T31 residue in MKK2 protein may play an important regulatory role in Musa spp under cold stress. (C) Dot blot of unmodified or phosphorylated MKK2 peptides with anti-MKK2 Thr31 antibody. (D) A rabbit polyclonal antibody against MKK2 intact protein and a rabbit polyclonal phospho-specific antibody against p-Thr31 of MKK2. Numbers indicate the western blotting signal intensities normalized to the total protein contents, using a novel Stain-free technology for total protein quantification72.