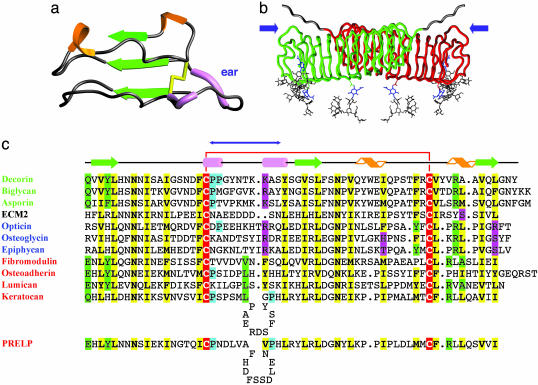
Fig. 2.
Topology and secondary structure of the C-terminal capping motif. (a) Ribbon diagram of the C-terminal capping motif of decorin. This motif includes LRR-XI, LRR-XII, and the additional C-terminal strand β13. Secondary structural elements are depicted as in Fig. 1. (b) Rope diagram of a glycosylated, extended model of the decorin dimer, showing the position of the two ear repeats (blue arrows). Extended oligosaccharide chains have been modeled (gray) onto the three N-acetylglucosamine residues (blue) to illustrate their approximate size and general positioning relative to the dimer surface. Both N termini also have been extended a few residues (gray) to illustrate the general sense of directionality. The GAG chains are not included in the model. (c) Structure-based alignment for the C-terminal motifs. Green text, class I SLRPs; red text, class II SLRPs; blue text, class III SLRPs. Yellow, conserved residues; red, conserved Cys residues; green, partially conserved residues; cyan, partially conserved Pro residues indicative of polyproline II conformation; magenta, polar residues occupying hallmark LRR hydrophobic sites. The blue double-headed arrow indicates the extent of the ear. Ear repeats in keratocan and PRELP are significantly longer than those for all other SLRPs and, therefore, are shown as containing insertion loops. The decorin sequence is that of the bovine protein; all others are of human proteins.