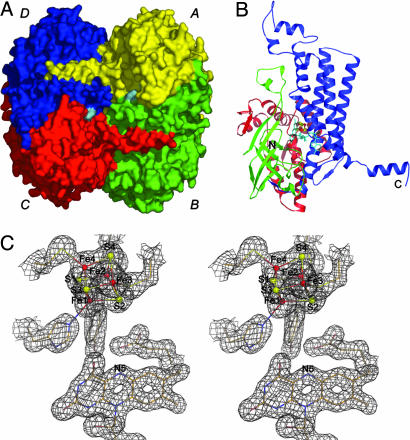
Fig. 1.
Crystal structure. (A) Surface representation of the 4-BUDH homotetramer. Each monomer's surface is individually colored, and the FAD's surface is shown in cyan. (B) Secondary structure topology of the monomer with the N-terminal domain (residues Met-1 to Leu-143) shown in red, the middle domain (residues Ile-144 to Gln-276) shown in green, and the C-terminal domain (residues Glu-277 to Lys-490) shown in blue. The [4Fe–4S]2+ cluster is shown as ball-and-sticks with Fe and S atoms shown in red and yellow, respectively. The FAD is displayed in sticks and shown in cyan. The N and C termini of the polypeptide chain are marked. (C) Example of the well defined electron density for the refined model (2 Fo - Fc electron density map contoured at 1.0 σ and shown in gray). Atom color code for protein residues and FAD is cream for carbon, red for oxygen, blue for nitrogen, and yellow for sulfur. Color code for the [4Fe–4S]2+ cluster is as in B. This figure and Fig. 2 were prepared with pymol (44).