Fig. 3.
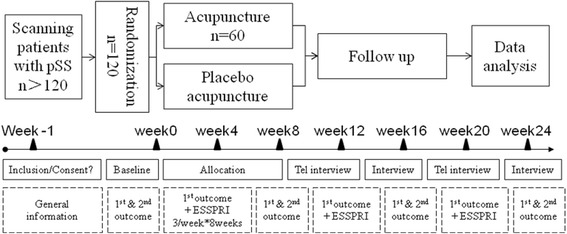
Trial flow & study design. General information = age, sex, weight, time when symptoms emerge, time when diagnosed, anti-SSA antibodies, abnormal Schirmer test results, decreased unstimulated salivary flow, previous systemic involvement, previous treatment with another immunosuppressant, current systemic involvement. 1st outcome = numeric analog scale (NAS) (Dryness, Pain and Fatigue). 2nd outcome = EULAR Sjogren Syndrome Patient Reported Index (ESSPRI), EULAR Sjogren Syndrome Disease Activity Index (ESSDAI), Medical Outcome Study Short From 36 Short-Form Health Survey (SF-36), Hospital Anxiety and Depression (HAD), Serum Immunoglobulin (IgG), IgA and IgM, Schirner test score and unstimulated salivary flow, salivary glands untrasounds
